Volume 7, Issue 4 (Journal of Clinical and Basic Research (JCBR) 2023)
jcbr 2023, 7(4): 5-9 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jahani G, Hojjati N. The relationship between coronavirus disease 2019 (COVID-19) vaccines, abnormal uterine bleeding, and hormonal disorders in women of reproductive age: one-center cross-sectional results. jcbr 2023; 7 (4) :5-9
URL: http://jcbr.goums.ac.ir/article-1-414-en.html
URL: http://jcbr.goums.ac.ir/article-1-414-en.html
1- Golestan University of Medical Sciences, Gorgan, Iran
2- Department of Obstetrics and Gynecology, Golestan University of Medical Sciences, Gorgan, Iran ,Dr.nhojjati@gmail.com
2- Department of Obstetrics and Gynecology, Golestan University of Medical Sciences, Gorgan, Iran ,
Keywords: Metrorrhagia, COVID-19 vaccines, Hormone dysfunctions, Sexual dysfunctions, Psychological, Reproductive history
Full-Text [PDF 578 kb]
(1006 Downloads)
| Abstract (HTML) (2771 Views)

Nausea and vomiting occurred in 2 women after the injection of the first dose of the vaccine. Fever occurred in 3 women after the first dose and in 1 woman after the injection of the second dose of the vaccine. Dizziness occurred in 1 woman after the first dose of the vaccine, and 1 woman mentioned a sore throat after the first dose. Drowsiness was reported by 1 woman after the first dose and 2 women after the second dose. Coughs in 4 patients occurred after the first dose and in 4 patients after the second dose. One person also mentioned early AUB (bleeding out of the regular cycle in 3 days after vaccination) in the second dose; she also had hair loss after the first dose. Moreover, 40 women did not mention any complications either after the first or the second dose. The other common complications were myalgia in 44 women after the second dose and 41 women after the first dose, hair loss in 10 women after the first dose and 7 after the second dose, headache in 31 subjects after the first dose, and in 16 subjects after the second dose. Moreover, 52 women (45.6%) had increased bleeding time, and 3 women (2.6%) had decreased bleeding time; 55 women (48.2%) had increased bleeding volume (of whom 4 women (3.5%) had clot discharge, and one woman (0.9%) only had clot discharge without any change in the bleeding volume). Besides, 12 women (10.5%) reported decreased bleeding volume. There were 47 women (41.2%) with an increased frequency and 8 women (7%) with a decreased frequency of bleeding (Table 7).
As mentioned, 1 woman suffered from thrombocytopenia, which increased the volume and frequency of bleeding, as expected. The change in the length, volume, and frequency of bleeding was measured with a checklist and based on the comparison of the individual with the previous trends of her menstruation. A subject had a final diagnosis of HPV (human papillomavirus) and presented with clinical symptoms of increased length of bleeding period and volume of bleeding.
We took into account the patients' ultrasounds; if they did not explain the symptoms, according to the chi-square test, the COVID-19 vaccination showed a significant relationship with the change in the bleeding process (P=0.049, df=1, value= 3.871). Nine women had associated hormonal disorders (4 had FSH above 12.4, 1 woman had TSH above 5, 1 woman had LH of 58, 3 had hyperprolactinemia), and also 6 had coagulation disorders and PTT of more than 35; there was no significant relationship between disorders with specific hormones and AUB. However, the presence of hormonal disorders with the chi-square test and the presence of AUB, despite increased PTT, had a significant relationship with AUB (respectively: [P=0.269, df=2, value=2.625] and [P=0.011 and df=1, value=6.474]).
According to the independent t-test, age in this study had no significant relationship with AUB (P=630., F=233.) A significant relationship between AUB and underlying diseases (anemia, hypothyroidism, obesity, diabetes, and hypertension) was observed in the chi-square test (P=088., df=1, value=2.908).
The history of COVID-19 infection with the development of AUB had a significant relationship according to the chi-square test. Had used, it was not enough to consider this relationship significant. Abnormal uterine bleeding (AUB) had a significant relationship with the AstraZeneca vaccine, and then Sinopharm, in the chi-square test, but this relationship was not observed with COVIran Barekat (P=612. in complications of first dose vaccine and 762. in complications of second dose and AUB; P=380).
The relationship between the type of vaccines and all three indicators (frequency, volume, and duration of bleeding) related to AUB was measured by the chi-square test, and all three showed a significant relationship (P for frequency = 0.13, P for duration = 453., and P for volume = 498.).
Discussion
Coronavirus, which caused a global epidemic 4 years ago, has been controlled to a great extent with the discovery of COVID-19 vaccines. Now, it is no longer a global problem, according to the World Health Organization. Some of these vaccines, which have been produced in different types by many countries and organizations, are out of order, while some have been effective. As expected, side effects have been reported by researchers in different parts of the world following the injection of COVID-19 vaccines.
Because the vaccines remain effective for a limited time, international monitoring organizations suggest additional doses and boosters. However, our country has not yet administered these vaccines to the public, unlike other developed nations. Due to the lack of reported and confirmed research, the production of Iranian COVID-19 vaccines can cause unexpected and ambiguous side effects.
In the current study, we explored the relationship between AUB and COVID-19 vaccines, which was significant. These relationships can be examined from several points of view: new hormonal relationships, altered relationships with blood indicators, problems for subsequent pregnancies, associations with specific vaccine types, and alterations in the uterine bleeding index.
Diabetes, hypothyroidism, high blood pressure, and lung diseases have been part of the risk factors for COVID-19 in various studies. In this study, there was a significant relationship between the complications and the presence of underlying diseases, and some underlying diseases were higher than expected from the normal population without complications; still, the number of available data is not sufficient to make a correct judgment. although it can be considered effective in reducing the capacity of people to tolerate possible complications caused by having underlying diseases. The average age of the subjects in this study was 41.12 years, which was completely consistent with the study by Asgari et al. (2021) in the same referral center (20).
Hormonal treatments, whether to prevent unwanted pregnancy or for other therapeutic effects, can change the level of hormones and are, therefore, a confounding factor for the identification of vaccines as an independent factor that affects abnormal bleeding. Although this study did not examine a large number of women under hormonal treatment, this factor is considered influential (21).
Due to its independent effect on bleeding, as shown by FIGO's AUB guideline in April 2020, COVID-19 infection was another factor that could have distorted the results of COVID-19 vaccination; nationwide vaccination was done during the pandemic and that receiving the vaccine was dependent on not having related symptoms and without a valid diagnostic method. Because of the sanctions on Iran and the fact that CT scans are more sensitive than PCR tests, it is difficult to distinguish diseases like influenza or the common cold from COVID-19 as they may have similar symptoms and require similar treatment. However, due to the reliable identification of symptoms and the fear of visiting hospitals or medical centers during the epidemic, as well as the preference for home remedies and alternative treatments, fewer people have sought medical help even if they are actually sick. Therefore, our study takes into account the presence of certain COVID-19 symptoms as there is a possibility of people being infected. Not needing to be hospitalized for more than 1 week and not needing mechanical ventilation during hospitalization after being infected with COVID-19 were the criteria for not severe infectious in people with a history so that the confounding effect of this disease could be reduced. The occurrence of complications after the injection of the second dose was very similar to the first dose, which can indicate the uniqueness of people in contracting with new medicines. The intervals of showing bleeding problems indicate long-term complications, although it is recommended that more extensive studies be conducted and more intervals be included to identify this relationship more precisely.
Hair loss and alopecia were among the symptoms that were considered to be related to sex hormones (22). Other studies expected a significant relationship between this symptom and changes in the level of sex hormones. Although paying attention to the point that the subjects present in the study met the entering criteria, most of the subjects did not mention the onset of these symptoms and visited later; and the level of hormones with a time difference has been measured, this relationship was not significant. However, the relationship between this symptom (alopecia) and the development of AUB was significant, which can indicate the possibility of the relationship between these two indicators, but requires more extensive studies.
Abnormal uterine bleeding is related to the level of hormones, coagulation indicators, the health status of women, and underlying diseases. It is also associated with social indicators, such as addiction, and various other factors that are more difficult to evaluate, such as stress levels and neural conditions. It affects people's mental status and even their nutritional status. The social stress level is higher during pandemics. Considering the rumors and reports about vaccines, the situation has been a little more complicated than expected. Nevertheless, the relationship between the type of vaccines and each AUB indicator has been significant, although this result still seems to have many confounding indicators and requires more studies (23 and 24).
In general, the side effects of the AstraZeneca vaccine were reported the most, followed by Sinopharm and, finally, Iranian vaccines. Still, the effectiveness of these vaccines differs, and the number of data is not sufficient to be able to make any conclusions about each vaccine. However, the injection of vaccines has a significant relationship with AUB (except for the change in each indicator because all three are significant). Besides, AUB has a significant relationship with PTT and hormonal changes. Whether vaccines show a precise relationship with hormonal changes or coagulation indices requires a different type of study.
Conclusion
The evaluation of side effects of COVID-19 vaccines is crucial, particularly in light of the recent stabilization of the pandemic. This assessment is linked to enhancing the efficacy of prevention and treatment measures, as well as gaining a deeper understanding of potential complications that may arise after vaccination for healthcare workers. Abnormal uterine bleeding is associated with many measurable indicators (hormonal levels, coagulation indicators, etc.), but some other indicators are difficult to measure (nutrition, stress level, level of fear of vaccines, etc.). It can indicate the general health of women in many ways. Natural uterine bleeding affects not only women's health but also the health of the future generations.
In our study, there was a significant relationship between the injection of vaccines and the occurrence of AUB regardless of hormonal disorders and coagulation disorders, which were more likely to occur in a wider time frame (6 months or more). In this study, there is an anticipation of health care workers being prepared to manage the side effects in individuals who have been vaccinated. At the same time, efforts are being made to address and minimize additional side effects. Although different types of vaccines can cause different effects, the long-term effects of these vaccines should be considered, especially in women with the possibility of hormonal changes and abnormal bleeding, and the use of less complicated vaccines is recommended.
Acknowledgement
We would like to extend our appreciation to everyone who participated in this study. I am also grateful to Golestan University of Medical Sciences for supporting the implementation and formation of this project.
Funding sources
The authors have no relationships with companies or received financial support for this study.
Ethical statement
This study was carried out with the approval of the Research Committee of Golestan University of Medical Sciences and after obtaining an ethics code (IR.GOUMS.REC.1402.004).
Conflicts of interest
The authors declare that they have no conflicts of interest related to this research study. Dr. Ghoncheh Jahani and Dr. Nazanin Hojjati have no financial or personal relationships that could potentially bias or influence the content and findings presented in this manuscript. This includes any financial relationships with pharmaceutical companies, biomedical device manufacturers, or other organizations that may have a direct or indirect interest in the subject matter discussed in the manuscript. The authors also confirm that no funding sources had any involvement in the study design, data collection, analysis, interpretation, writing, or decision to submit this manuscript for publication.
Author contributions
Dr. Ghoncheh Jahani contributed significantly to the study by serving as the principal researcher and data collector. She designed the study, collected and analyzed the data, and wrote the manuscript. She also provided critical feedback and revisions to the manuscript.
Dr. Nazanin Hojjati played a critical role in the study as the supervisor and editor. She led the study, provided guidance on study design and data analysis, and reviewed and edited the manuscript. She also provided valuable feedback and suggestions for improving the manuscript.
Full-Text: (582 Views)
Introduction
Since the coronavirus disease 2019 (COVID-19) was first observed in 2019 in Wuhan, China (1), many studies have been conducted on the coronavirus infection and its complications. Severe complications such as thrombotic complications, cardiac complications, acute kidney failure, gastrointestinal symptoms, damage to liver cells, hyperglycemia, diabetic ketoacidosis, neurological diseases, eye symptoms, skin complications, and alopecia have been recorded after contracting the virus (2 and 3, 4 and 6). In many patients, multisystem complications, such as cardiovascular and digestive problems, have also been reported (5). Since the beginning of vaccination in early December 2020 (7), there have been various reports of menstrual changes (13). Following this pandemic, most countries, including Iran, started injecting vaccines (9). These vaccines are based on different bases, such as messenger ribonucleic acid (mRNA) (Pfizer and Moderna), the virus vector (AstraZeneca, Sputnik), or a weakened or killed virus (Sinopharm, COVIran Barekat). Along with all their advantages, in a percentage of people, these vaccines caused various side effects, such as headaches, fatigue, myalgia, fever, thrombocytopenia, and cerebral venous sinus thrombosis (CVST). These symptoms might appear immediately after vaccination and resolve in a short time or appear long after the injection of the vaccine (10, 11, 18, 12). In some studies, up to 50-60% of women infected with COVID-19 during the first round of vaccination and 60-70% in the second round experience menstrual changes regardless of the type of vaccine and the menstrual cycle phase they were in; these changes included polymenorrhea and oligomenorrhea, hypermenorrhea and hypomenorrhea, and abnormal uterine bleeding (AUB) (8,13). Moreover, health centers have reported an increase in the number of clients due to menstrual changes and AUB based on the relationship between the vaccine and menstrual changes (13, 14). Abnormal uterine bleeding is one of the most common complaints of clients to gynecologists. It is defined based on changes in the volume, frequency, and duration of bleeding (17 and 19). In the past, the definition of AUB included a wide range of symptoms of different etiologies, but in 2011, the classification of the International Federation Of Gynecology and Obstetrics (FIGO), i.e., PALM-COEIN (polyp, adenomyosis, leiomyoma, malignancy, and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not yet classified) has been introduced to determine the etiology of AUB (Pregnancy-related bleeding is not included in this definition) (15,16). Different types of AUB, which are divided based on etiology, can have specific and nonspecific signs and symptoms. For example, AUB-O, which leads to a chronic decrease in ovulation and progesterone, according to the etiology and the hormonal disorder responsible for the lack of ovulation, can lead to unpredictable, heavy, or prolonged bleeding (17). Although the studies point to the possibility of changes in the pattern of abnormal monthly bleeding, so far, the background of these changes and the possibility of hormonal changes have not been discussed. Therefore, this study aimed to find the relationship between COVID-19 vaccination and hormonal disorders and the relationship between these disorders and the prevalence of AUB in women of reproductive age.
Methods
This cross-sectional study was conducted on 114 patients referred to the Gynecology Center of Sayad Shirazi Hospital, Gorgan (Iran), from March to May 2023. The information of the patients was collected by the doctors and assistants present in the clinics and reported. The sampling method was a simple census, and all the eligible patients were included.
Inclusion criteria:
Since the coronavirus disease 2019 (COVID-19) was first observed in 2019 in Wuhan, China (1), many studies have been conducted on the coronavirus infection and its complications. Severe complications such as thrombotic complications, cardiac complications, acute kidney failure, gastrointestinal symptoms, damage to liver cells, hyperglycemia, diabetic ketoacidosis, neurological diseases, eye symptoms, skin complications, and alopecia have been recorded after contracting the virus (2 and 3, 4 and 6). In many patients, multisystem complications, such as cardiovascular and digestive problems, have also been reported (5). Since the beginning of vaccination in early December 2020 (7), there have been various reports of menstrual changes (13). Following this pandemic, most countries, including Iran, started injecting vaccines (9). These vaccines are based on different bases, such as messenger ribonucleic acid (mRNA) (Pfizer and Moderna), the virus vector (AstraZeneca, Sputnik), or a weakened or killed virus (Sinopharm, COVIran Barekat). Along with all their advantages, in a percentage of people, these vaccines caused various side effects, such as headaches, fatigue, myalgia, fever, thrombocytopenia, and cerebral venous sinus thrombosis (CVST). These symptoms might appear immediately after vaccination and resolve in a short time or appear long after the injection of the vaccine (10, 11, 18, 12). In some studies, up to 50-60% of women infected with COVID-19 during the first round of vaccination and 60-70% in the second round experience menstrual changes regardless of the type of vaccine and the menstrual cycle phase they were in; these changes included polymenorrhea and oligomenorrhea, hypermenorrhea and hypomenorrhea, and abnormal uterine bleeding (AUB) (8,13). Moreover, health centers have reported an increase in the number of clients due to menstrual changes and AUB based on the relationship between the vaccine and menstrual changes (13, 14). Abnormal uterine bleeding is one of the most common complaints of clients to gynecologists. It is defined based on changes in the volume, frequency, and duration of bleeding (17 and 19). In the past, the definition of AUB included a wide range of symptoms of different etiologies, but in 2011, the classification of the International Federation Of Gynecology and Obstetrics (FIGO), i.e., PALM-COEIN (polyp, adenomyosis, leiomyoma, malignancy, and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not yet classified) has been introduced to determine the etiology of AUB (Pregnancy-related bleeding is not included in this definition) (15,16). Different types of AUB, which are divided based on etiology, can have specific and nonspecific signs and symptoms. For example, AUB-O, which leads to a chronic decrease in ovulation and progesterone, according to the etiology and the hormonal disorder responsible for the lack of ovulation, can lead to unpredictable, heavy, or prolonged bleeding (17). Although the studies point to the possibility of changes in the pattern of abnormal monthly bleeding, so far, the background of these changes and the possibility of hormonal changes have not been discussed. Therefore, this study aimed to find the relationship between COVID-19 vaccination and hormonal disorders and the relationship between these disorders and the prevalence of AUB in women of reproductive age.
Methods
This cross-sectional study was conducted on 114 patients referred to the Gynecology Center of Sayad Shirazi Hospital, Gorgan (Iran), from March to May 2023. The information of the patients was collected by the doctors and assistants present in the clinics and reported. The sampling method was a simple census, and all the eligible patients were included.
Inclusion criteria:
- Patients who reached menarche and did not have menopausal symptoms (no menstruation for 12 consecutive months) and were 9 to 65 years old
- Women who received at least 1 dose of a COVID-19 vaccine
- Women who had AUB in the past 12 months and a change in bleeding pattern after vaccine injection (Due to the impact of other disorders, including hormonal disorders, thyroid disorders, prolactin disorders, obesity, and polycystic ovary syndrome, the use of drugs, especially contraceptive methods, the patients would be included if there was a change in the bleeding process (blood volume, length of menstruation, frequency of menstruation) and if the client had clearly noticed the change.)
- Women who were willing to cooperate
Exclusion criteria:
- Unwillingness to participation
- Incomplete information
- Previous history of AUB within 12 months before the first dose of the COVID-19 vaccine
- Having an ultrasound justifying the symptoms in the last 12 months
- Pregnancy
The doctor first recorded the demographic data (age, marital status, number of children) and took a complete history of the patients in terms of underlying diseases, hormone therapy, the use of contraceptive methods and their type, the first day of the menstrual cycle, menstruation status before and after each vaccine injection, and the history of COVID-19 infection.
Symptoms of infection with COVID-19, date of infection, duration of infection, severity of the disease in terms of symptoms such as fever and chills, cough, shortness of breath, hospitalization, length of hospitalization, and the need to use a ventilator were also recorded.
The vaccination history of the patients was checked, which included the type of vaccine and the date of the first and second vaccine injections.
The side effects of vaccine injection included headache, fatigue, muscle pain, fever and chills, lethargy, thrombocytopenia, and CVST. The time when these side effects occurred after the injection was also recorded.
Menstrual disorders following the injection of the vaccine were investigated, e.g., the length of the menses, changes compared to the previous menstruations, including the volume, length, and frequency of bleeding and spotting (number in months) during the period, and how long after the injection these symptoms appeared and how long they lasted.
The hormonal profiles of the patients included:
1. Human chorionic gonadotropin (HCG)
2. Follicle-stimulating hormone (FSH), which is checked during the follicular phase of the menstrual cycle
3. Thyroid-stimulating hormone (TSH)
4. Prolactin
5. Estradiol
Due to the connection between COVID-19 vaccination and blood disorders, a complete blood cell count (CBC) was also requested. In addition to the aforementioned tests, ultrasound and B-Hcg (beta-human chorionic gonadotropin) serum titer (performed in patients without fasting and upon arrival with the blood test), FSH (checked during the follicular phase of the menstrual cycle), TSH, prolactin, and estradiol were requested during the follow-up to find out the cause of AUB and check for the presence of underlying pathology.
The recorded data were analyzed in SPSS vs. 16 (SPSS Inc., Chicago, IL, USA), and the possible relationships between the data, complications, hormonal profiles, COVID-19, and the vaccines used were investigated.
The FIGO classification was used in this study to describe AUB (15). Due to the general inconsistency in the nomenclature used to describe AUB and the classification for AUB etiology, this classification was proposed to consider PALM-COEIN (polyp, adenomyosis, leiomyoma, malignancy, and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not yet classified). The classification was approved by the FIGO. In this classification, a checklist including the following items is used to describe AUB:
Symptoms of infection with COVID-19, date of infection, duration of infection, severity of the disease in terms of symptoms such as fever and chills, cough, shortness of breath, hospitalization, length of hospitalization, and the need to use a ventilator were also recorded.
The vaccination history of the patients was checked, which included the type of vaccine and the date of the first and second vaccine injections.
The side effects of vaccine injection included headache, fatigue, muscle pain, fever and chills, lethargy, thrombocytopenia, and CVST. The time when these side effects occurred after the injection was also recorded.
Menstrual disorders following the injection of the vaccine were investigated, e.g., the length of the menses, changes compared to the previous menstruations, including the volume, length, and frequency of bleeding and spotting (number in months) during the period, and how long after the injection these symptoms appeared and how long they lasted.
The hormonal profiles of the patients included:
1. Human chorionic gonadotropin (HCG)
2. Follicle-stimulating hormone (FSH), which is checked during the follicular phase of the menstrual cycle
3. Thyroid-stimulating hormone (TSH)
4. Prolactin
5. Estradiol
Due to the connection between COVID-19 vaccination and blood disorders, a complete blood cell count (CBC) was also requested. In addition to the aforementioned tests, ultrasound and B-Hcg (beta-human chorionic gonadotropin) serum titer (performed in patients without fasting and upon arrival with the blood test), FSH (checked during the follicular phase of the menstrual cycle), TSH, prolactin, and estradiol were requested during the follow-up to find out the cause of AUB and check for the presence of underlying pathology.
The recorded data were analyzed in SPSS vs. 16 (SPSS Inc., Chicago, IL, USA), and the possible relationships between the data, complications, hormonal profiles, COVID-19, and the vaccines used were investigated.
The FIGO classification was used in this study to describe AUB (15). Due to the general inconsistency in the nomenclature used to describe AUB and the classification for AUB etiology, this classification was proposed to consider PALM-COEIN (polyp, adenomyosis, leiomyoma, malignancy, and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not yet classified). The classification was approved by the FIGO. In this classification, a checklist including the following items is used to describe AUB:
- Frequency (24 to 38 days is normal.)
- Length of bleeding (up to 8 days is normal.)
- Bleeding regularity (The shortest to the longest period should be at least 9 days apart to consider it a regular cycle.)
- Bleeding volume
Limitations of this study:
- Patients' difficulty in accurately remembering their complications
- Simultaneous infection with COVID-19 and vaccine injection
- Visiting patients during menstruation
- Different doctors visit and evaluate the patients
- Reasons for hormonal disorders except for vaccination and coincidences
The data were analyzed in SPSS v. 16 (SPSS Inc., Chicago, IL, USA) by the chi-square, independent t-test, and analysis of variance (ANOVA). The chi-square test was used for qualitative data, and the two other tests were used for examining the associations between quantitative and qualitative data. The significance level of the tests was considered 0.05 in this study.
The data were collected after obtaining the necessary permission from the Research Center and Research Committee of Golestan University of Medical Sciences (IR.GOUMS.REC.1402.004).
During this study, all the data obtained were without personal identity information. After we obtained the participants' verbal consent, we recorded their information, and if a referent declined participation, we would not fill out the form. The tests that were performed for the patients were routine tests requested to follow up on AUB, and no additional tests were requested for this study. The information is kept with the doctor and will only be used for this study.
Results
A total of 114 people were included through a simple census with defined criteria. The participants had an average age of 41.12 ± 7.035, with a minimum of 23 years and a maximum of 65 years. (Figure 1).

Among the 114 participants, 27 (23.7%) reported AUB in the 12 months before the injection of the first dose of the vaccine, although these people had obvious changes in the pattern of uterine bleeding.
Moreover, 36 women (31.6%) did not have any underlying disease, while diabetes (18.42%), high blood pressure (25.43%), and hypothyroidism (17.54%) had higher percentages among others (Table 1).
One woman (0.8%) was undergoing hormonal treatment with progesterone, except for the use of hormones to prevent pregnancy. Examination of contraceptive methods indicated the use of pills containing estrogen and progesterone in 8 (7%) and pills with low-dose (LD) estrogen in 5.3% of the women (Table 2).
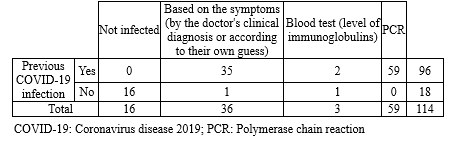
Besides, 96 women (84.2%) mentioned a history of coronavirus infection, of whom 59 people (61.45%) were confirmed by polymerase chain reaction (PCR), and 3 (3.2% of the infected people) were confirmed by blood analysis (Table 3).
There was 1 patient with a history of thrombocytopenia among the women who reported a previous coronavirus infection. Three-quarters (75%) of the women noticed a change in their bleeding status. The symptoms caused were coughs (86.45%), sore throat (58.33%), and fever (53.12%) (Table 4).
Furthermore, 14 women (12.28% of all the women and 14.58% of those with a history of COVID-19) were hospitalized due to COVID-19. None of them needed mechanical ventilation, and the average length of hospitalization days was 5 ±1.748 days, with a minimum length of 2 days and a maximum length of 7 days. Most people in this study had received more than one dose of vaccine (48.24% had received two doses, and 42.1% had received three doses of vaccine) (Table 5).
In addition, 42 women (37.5%) had the same side effects after the first and second vaccines (including 20 women (27.77% of those with complications) who had the same complications after the first vaccine injection with the second vaccine injection, or they had shown same complications in addition to some new complications in other injection (22 other people or 19.64% of people with complications). Despite the significant relationship between the complications created according to the chi-square test after the injection of the first and the second vaccines, the number of findings was not enough to rely on the obtained result (Table 6).
The data were collected after obtaining the necessary permission from the Research Center and Research Committee of Golestan University of Medical Sciences (IR.GOUMS.REC.1402.004).
During this study, all the data obtained were without personal identity information. After we obtained the participants' verbal consent, we recorded their information, and if a referent declined participation, we would not fill out the form. The tests that were performed for the patients were routine tests requested to follow up on AUB, and no additional tests were requested for this study. The information is kept with the doctor and will only be used for this study.
Results
A total of 114 people were included through a simple census with defined criteria. The participants had an average age of 41.12 ± 7.035, with a minimum of 23 years and a maximum of 65 years. (Figure 1).

Among the 114 participants, 27 (23.7%) reported AUB in the 12 months before the injection of the first dose of the vaccine, although these people had obvious changes in the pattern of uterine bleeding.
Moreover, 36 women (31.6%) did not have any underlying disease, while diabetes (18.42%), high blood pressure (25.43%), and hypothyroidism (17.54%) had higher percentages among others (Table 1).
One woman (0.8%) was undergoing hormonal treatment with progesterone, except for the use of hormones to prevent pregnancy. Examination of contraceptive methods indicated the use of pills containing estrogen and progesterone in 8 (7%) and pills with low-dose (LD) estrogen in 5.3% of the women (Table 2).
Besides, 96 women (84.2%) mentioned a history of coronavirus infection, of whom 59 people (61.45%) were confirmed by polymerase chain reaction (PCR), and 3 (3.2% of the infected people) were confirmed by blood analysis (Table 3).
There was 1 patient with a history of thrombocytopenia among the women who reported a previous coronavirus infection. Three-quarters (75%) of the women noticed a change in their bleeding status. The symptoms caused were coughs (86.45%), sore throat (58.33%), and fever (53.12%) (Table 4).
Furthermore, 14 women (12.28% of all the women and 14.58% of those with a history of COVID-19) were hospitalized due to COVID-19. None of them needed mechanical ventilation, and the average length of hospitalization days was 5 ±1.748 days, with a minimum length of 2 days and a maximum length of 7 days. Most people in this study had received more than one dose of vaccine (48.24% had received two doses, and 42.1% had received three doses of vaccine) (Table 5).
In addition, 42 women (37.5%) had the same side effects after the first and second vaccines (including 20 women (27.77% of those with complications) who had the same complications after the first vaccine injection with the second vaccine injection, or they had shown same complications in addition to some new complications in other injection (22 other people or 19.64% of people with complications). Despite the significant relationship between the complications created according to the chi-square test after the injection of the first and the second vaccines, the number of findings was not enough to rely on the obtained result (Table 6).
Table 1. Underlying diseases |

Table 4. Symptoms during COVID-19 infection (frequencies) |
|
Table 5. Frequency of vaccine injections
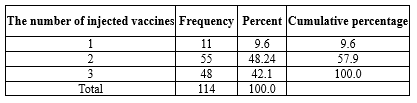 |
Table 6. Number and type of the injected vaccines |

Nausea and vomiting occurred in 2 women after the injection of the first dose of the vaccine. Fever occurred in 3 women after the first dose and in 1 woman after the injection of the second dose of the vaccine. Dizziness occurred in 1 woman after the first dose of the vaccine, and 1 woman mentioned a sore throat after the first dose. Drowsiness was reported by 1 woman after the first dose and 2 women after the second dose. Coughs in 4 patients occurred after the first dose and in 4 patients after the second dose. One person also mentioned early AUB (bleeding out of the regular cycle in 3 days after vaccination) in the second dose; she also had hair loss after the first dose. Moreover, 40 women did not mention any complications either after the first or the second dose. The other common complications were myalgia in 44 women after the second dose and 41 women after the first dose, hair loss in 10 women after the first dose and 7 after the second dose, headache in 31 subjects after the first dose, and in 16 subjects after the second dose. Moreover, 52 women (45.6%) had increased bleeding time, and 3 women (2.6%) had decreased bleeding time; 55 women (48.2%) had increased bleeding volume (of whom 4 women (3.5%) had clot discharge, and one woman (0.9%) only had clot discharge without any change in the bleeding volume). Besides, 12 women (10.5%) reported decreased bleeding volume. There were 47 women (41.2%) with an increased frequency and 8 women (7%) with a decreased frequency of bleeding (Table 7).
As mentioned, 1 woman suffered from thrombocytopenia, which increased the volume and frequency of bleeding, as expected. The change in the length, volume, and frequency of bleeding was measured with a checklist and based on the comparison of the individual with the previous trends of her menstruation. A subject had a final diagnosis of HPV (human papillomavirus) and presented with clinical symptoms of increased length of bleeding period and volume of bleeding.
We took into account the patients' ultrasounds; if they did not explain the symptoms, according to the chi-square test, the COVID-19 vaccination showed a significant relationship with the change in the bleeding process (P=0.049, df=1, value= 3.871). Nine women had associated hormonal disorders (4 had FSH above 12.4, 1 woman had TSH above 5, 1 woman had LH of 58, 3 had hyperprolactinemia), and also 6 had coagulation disorders and PTT of more than 35; there was no significant relationship between disorders with specific hormones and AUB. However, the presence of hormonal disorders with the chi-square test and the presence of AUB, despite increased PTT, had a significant relationship with AUB (respectively: [P=0.269, df=2, value=2.625] and [P=0.011 and df=1, value=6.474]).
|
Table 7. Abnormal uterine bleeding (AUB) types
 |
The history of COVID-19 infection with the development of AUB had a significant relationship according to the chi-square test. Had used, it was not enough to consider this relationship significant. Abnormal uterine bleeding (AUB) had a significant relationship with the AstraZeneca vaccine, and then Sinopharm, in the chi-square test, but this relationship was not observed with COVIran Barekat (P=612. in complications of first dose vaccine and 762. in complications of second dose and AUB; P=380).
The relationship between the type of vaccines and all three indicators (frequency, volume, and duration of bleeding) related to AUB was measured by the chi-square test, and all three showed a significant relationship (P for frequency = 0.13, P for duration = 453., and P for volume = 498.).
Discussion
Coronavirus, which caused a global epidemic 4 years ago, has been controlled to a great extent with the discovery of COVID-19 vaccines. Now, it is no longer a global problem, according to the World Health Organization. Some of these vaccines, which have been produced in different types by many countries and organizations, are out of order, while some have been effective. As expected, side effects have been reported by researchers in different parts of the world following the injection of COVID-19 vaccines.
Because the vaccines remain effective for a limited time, international monitoring organizations suggest additional doses and boosters. However, our country has not yet administered these vaccines to the public, unlike other developed nations. Due to the lack of reported and confirmed research, the production of Iranian COVID-19 vaccines can cause unexpected and ambiguous side effects.
In the current study, we explored the relationship between AUB and COVID-19 vaccines, which was significant. These relationships can be examined from several points of view: new hormonal relationships, altered relationships with blood indicators, problems for subsequent pregnancies, associations with specific vaccine types, and alterations in the uterine bleeding index.
Diabetes, hypothyroidism, high blood pressure, and lung diseases have been part of the risk factors for COVID-19 in various studies. In this study, there was a significant relationship between the complications and the presence of underlying diseases, and some underlying diseases were higher than expected from the normal population without complications; still, the number of available data is not sufficient to make a correct judgment. although it can be considered effective in reducing the capacity of people to tolerate possible complications caused by having underlying diseases. The average age of the subjects in this study was 41.12 years, which was completely consistent with the study by Asgari et al. (2021) in the same referral center (20).
Hormonal treatments, whether to prevent unwanted pregnancy or for other therapeutic effects, can change the level of hormones and are, therefore, a confounding factor for the identification of vaccines as an independent factor that affects abnormal bleeding. Although this study did not examine a large number of women under hormonal treatment, this factor is considered influential (21).
Due to its independent effect on bleeding, as shown by FIGO's AUB guideline in April 2020, COVID-19 infection was another factor that could have distorted the results of COVID-19 vaccination; nationwide vaccination was done during the pandemic and that receiving the vaccine was dependent on not having related symptoms and without a valid diagnostic method. Because of the sanctions on Iran and the fact that CT scans are more sensitive than PCR tests, it is difficult to distinguish diseases like influenza or the common cold from COVID-19 as they may have similar symptoms and require similar treatment. However, due to the reliable identification of symptoms and the fear of visiting hospitals or medical centers during the epidemic, as well as the preference for home remedies and alternative treatments, fewer people have sought medical help even if they are actually sick. Therefore, our study takes into account the presence of certain COVID-19 symptoms as there is a possibility of people being infected. Not needing to be hospitalized for more than 1 week and not needing mechanical ventilation during hospitalization after being infected with COVID-19 were the criteria for not severe infectious in people with a history so that the confounding effect of this disease could be reduced. The occurrence of complications after the injection of the second dose was very similar to the first dose, which can indicate the uniqueness of people in contracting with new medicines. The intervals of showing bleeding problems indicate long-term complications, although it is recommended that more extensive studies be conducted and more intervals be included to identify this relationship more precisely.
Hair loss and alopecia were among the symptoms that were considered to be related to sex hormones (22). Other studies expected a significant relationship between this symptom and changes in the level of sex hormones. Although paying attention to the point that the subjects present in the study met the entering criteria, most of the subjects did not mention the onset of these symptoms and visited later; and the level of hormones with a time difference has been measured, this relationship was not significant. However, the relationship between this symptom (alopecia) and the development of AUB was significant, which can indicate the possibility of the relationship between these two indicators, but requires more extensive studies.
Abnormal uterine bleeding is related to the level of hormones, coagulation indicators, the health status of women, and underlying diseases. It is also associated with social indicators, such as addiction, and various other factors that are more difficult to evaluate, such as stress levels and neural conditions. It affects people's mental status and even their nutritional status. The social stress level is higher during pandemics. Considering the rumors and reports about vaccines, the situation has been a little more complicated than expected. Nevertheless, the relationship between the type of vaccines and each AUB indicator has been significant, although this result still seems to have many confounding indicators and requires more studies (23 and 24).
In general, the side effects of the AstraZeneca vaccine were reported the most, followed by Sinopharm and, finally, Iranian vaccines. Still, the effectiveness of these vaccines differs, and the number of data is not sufficient to be able to make any conclusions about each vaccine. However, the injection of vaccines has a significant relationship with AUB (except for the change in each indicator because all three are significant). Besides, AUB has a significant relationship with PTT and hormonal changes. Whether vaccines show a precise relationship with hormonal changes or coagulation indices requires a different type of study.
Conclusion
The evaluation of side effects of COVID-19 vaccines is crucial, particularly in light of the recent stabilization of the pandemic. This assessment is linked to enhancing the efficacy of prevention and treatment measures, as well as gaining a deeper understanding of potential complications that may arise after vaccination for healthcare workers. Abnormal uterine bleeding is associated with many measurable indicators (hormonal levels, coagulation indicators, etc.), but some other indicators are difficult to measure (nutrition, stress level, level of fear of vaccines, etc.). It can indicate the general health of women in many ways. Natural uterine bleeding affects not only women's health but also the health of the future generations.
In our study, there was a significant relationship between the injection of vaccines and the occurrence of AUB regardless of hormonal disorders and coagulation disorders, which were more likely to occur in a wider time frame (6 months or more). In this study, there is an anticipation of health care workers being prepared to manage the side effects in individuals who have been vaccinated. At the same time, efforts are being made to address and minimize additional side effects. Although different types of vaccines can cause different effects, the long-term effects of these vaccines should be considered, especially in women with the possibility of hormonal changes and abnormal bleeding, and the use of less complicated vaccines is recommended.
Acknowledgement
We would like to extend our appreciation to everyone who participated in this study. I am also grateful to Golestan University of Medical Sciences for supporting the implementation and formation of this project.
Funding sources
The authors have no relationships with companies or received financial support for this study.
Ethical statement
This study was carried out with the approval of the Research Committee of Golestan University of Medical Sciences and after obtaining an ethics code (IR.GOUMS.REC.1402.004).
Conflicts of interest
The authors declare that they have no conflicts of interest related to this research study. Dr. Ghoncheh Jahani and Dr. Nazanin Hojjati have no financial or personal relationships that could potentially bias or influence the content and findings presented in this manuscript. This includes any financial relationships with pharmaceutical companies, biomedical device manufacturers, or other organizations that may have a direct or indirect interest in the subject matter discussed in the manuscript. The authors also confirm that no funding sources had any involvement in the study design, data collection, analysis, interpretation, writing, or decision to submit this manuscript for publication.
Author contributions
Dr. Ghoncheh Jahani contributed significantly to the study by serving as the principal researcher and data collector. She designed the study, collected and analyzed the data, and wrote the manuscript. She also provided critical feedback and revisions to the manuscript.
Dr. Nazanin Hojjati played a critical role in the study as the supervisor and editor. She led the study, provided guidance on study design and data analysis, and reviewed and edited the manuscript. She also provided valuable feedback and suggestions for improving the manuscript.
Article Type: Research |
Subject:
Obstetrics and Gynecology
References
1. Han Y, Yang H. The transmission and diagnosis of 2019 novel coronavirus infection disease (COVID‐ 19): A Chinese perspective. J Med Virol. 2020;92(6):639-44. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. COVID-19 Coronavirus Pandemic. Worldometer; 2023. [View at Publisher]
3. Chen Y, Peng H, Wang L, Zhao Y, Zeng L, Gao H, et al. Infants born to mothers with a new coronavirus (COVID-19). Front Pediatr. 2020;8:104. [View at Publisher] [DOI] [PubMed] [Google Scholar]
4. Nguyen B, Tosti A. Alopecia in patients with COVID-19: A systematic review and meta-analysis. JAAD Int. 2022;7:67-77. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-13. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708-20. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. WHO. Coronavirus disease (COVID-19): Vaccines and vaccine safety.2023. [View at Publisher]
8. Li K, Chen G, Hou H, Liao Q, Chen J, Bai H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42(1):260-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Fathizadeh H, Afshar S, Masoudi MR, Gholizadeh P, Asgharzadeh M, Ganbarov K, et al. SARS-CoV-2 (Covid-19) vaccines structure, mechanisms and effectiveness: a review. Int J Biol Macromol. 2021:188:740-50. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Hernandez AF, Calina D, Poulas K, Docea AO, Tsatsakis AM. Safety of COVID19 vaccines administered in the EU: should we be concerned? Toxicol Rep. 2021;8:871-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Ostovan VR, Foroughi R, Rostami M, Almasi-Dooghaee M, Esmaili M, Bidaki AA, et al., Cerebral venous sinus thrombosis associated with COVID-19: a case series and literature review. J Neurol. 2021;268(10):3549-60. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, et al., US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26. COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448-56. [View at Publisher] [Google Scholar] [DOI] [PMID] [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Laganà AS, Veronesi G, Ghezzi F, Ferrario MM, Cromi A, Bizzarri M, et al. Evaluation of menstrual irregularities after COVID-19 vaccination: Results of the MECOVAC survey. Open Med (Wars). 2022;17(1):475-84. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. 14) Male V. Menstrual changes after covid-19 vaccination. BMJ. 2021;374:n2211. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Eunice Kennedy Shriver National Institute of Child Health and Human Development. Item of interest: NIH funds studies to assess potential effects of COVID-19 vaccination on menstruation. 2021. [View at Publisher] [Google Scholar]
16. Munro MG, Critchley HOD, Fraser IS. The FIGO classification of causes of abnormal uterine bleeding: Malcolm G. Munro, Hilary O.D. Crithcley, Ian S. Fraser, for the FIGO Working Group on Menstrual Disorders. Int J Gynaecol Obstet. 2011;113(1):1-2. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Munro MG, Critchley HOD, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113(1):3-13. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Fraser IS, Critchley HO, Broder M, Munro MG. The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. 2011;29(5):383-90. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Jones K, Sung S. Anovulatory Bleeding. Treasure Island (FL): StatPearls Publishing; 2023. [View at Publisher] [PMID] [Google Scholar]
20. Han X, Xu P, Ye Q. Analysis of COVID-19 vaccines: Types, thoughts, and application. J Clin Lab Anal. 2021;35(9):e23937. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Shapley M, Jordan K, Croft PR. An epidemiological survey of symptoms of menstrual loss in the community. Br J Gen Pract. 2004;54(502):359-63. [View at Publisher] [PMID] [Google Scholar]
22. Edelman A, Boniface ER, Benhar E, Han L, Matteson KA, Favaro C, et al. Association Between Menstrual Cycle Length and Coronavirus Disease 2019 (COVID-19) Vaccination: A U.S. Cohort. Obstet Gynecol. 2022;139(4):481-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Quejada LR, Wills MFT, Martínez-Ávila MC, Patiño-Aldana AF. Menstrual cycle disturbances after COVID-19 vaccination Luisa Rodríguez Quejada et al. Womens Health (Lond). 2022;18:17455057221109375. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Nazir M, Asghar S, Rathore MA, Shahzad A, Shahid A, Ashraf Khan A, et al. Menstrual abnormalities after COVID-19 vaccines: A systematic review. Vacunas. 2022;23:S77-87. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Lebar V, Laganà AS, Chiantera V, Kunič T, Lukanović D. The Effect of COVID-19 on the Menstrual Cycle: A Systematic Review. J Clin Med. 2022;11(13):3800. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Muhaidat N, Alshrouf MA, Azzam MI, Karam AM, Al-Nazer MW, Al-Ani A. Menstrual Symptoms After COVID-19 Vaccine: A Cross-Sectional Investigation in the MENA Region Nadia Muhaidat et al. Int J Womens Health. 2022;14:395-404. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Sarfraz A, Sarfraz Z, Sarfraz M, Nadeem Z, Felix M, Cherrez-Ojeda I. Menstrual irregularities following COVID-19 vaccination: A global cross-sectional survey. Ann Med Surg (Lond). 2022;81:104220. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Asgari Z, Hosseini R, Azadehrah M, Azadehrah M. Evaluation of the compatibility of ultrasound and hysteroscopic findings and histopathological results in women with abnormal uterine bleeding (AUB). Pakistan journal of Medical and Health Sciences.2021;15(1):633-40. [View at Publisher] [Google Scholar]
29. Jordahl- Iafrato MA, Reed H, Hadley SK, Kolman KB. A systematic approach to chronic abnormal uterine bleeding. J Fam Pract. 2019;68(2):82;84;86;92. [View at Publisher] [PMID] [Google Scholar]
30. Eyada MMK, Abd-Elhamid AAS, Elboghdady RAF, Gadallah AM, Azab M. Assessment of Female Sexual Dysfunction in Patients with Premenopausal Female Pattern Hair Loss. Advances in Sexual Medicine.2020;10(3):86-103. [View at Publisher] [DOI] [Google Scholar]
31. Michael Price T. Abnormal (Dysfunctional) Uterine Bleeding. Medscape. 2022. [View at Publisher]
32. Kanagasabai PS, Filoche S, Grainger R, Henry C, Hay-Smith J. Interventions to improve access to care for abnormal uterine bleeding: A systematic scoping review. Int J Gynecol Obstet. 2023;160(1):38-48. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







