Volume 8, Issue 2 (Journal of Clinical and Basic Research (JCBR) 2024)
jcbr 2024, 8(2): 17-19 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mahfouz N N, Abd El-Shaheed A, Sallam S F, Moustafa R S I, El-Zayat S R, Sibaii H. Obesity induced renal injury: Could It be detected early?. jcbr 2024; 8 (2) :17-19
URL: http://jcbr.goums.ac.ir/article-1-408-en.html
URL: http://jcbr.goums.ac.ir/article-1-408-en.html
Nermine Nabil Mahfouz1 

 , Azza Abd El-Shaheed1
, Azza Abd El-Shaheed1 

 , Sara Fawzy Sallam *2
, Sara Fawzy Sallam *2 

 , Rehab Selim Ismail Moustafa1
, Rehab Selim Ismail Moustafa1 

 , Salwa Refaat El-Zayat3
, Salwa Refaat El-Zayat3 

 , Hiba Sibaii3
, Hiba Sibaii3 




 , Azza Abd El-Shaheed1
, Azza Abd El-Shaheed1 

 , Sara Fawzy Sallam *2
, Sara Fawzy Sallam *2 

 , Rehab Selim Ismail Moustafa1
, Rehab Selim Ismail Moustafa1 

 , Salwa Refaat El-Zayat3
, Salwa Refaat El-Zayat3 

 , Hiba Sibaii3
, Hiba Sibaii3 


1- Department of Child Health, Medical Research and Clinical Studies Institute, Medical Research Centre of Excellence (MRCE), National Research Centre, Cairo, Egypt
2- Department of Child Health, Medical Research and Clinical Studies Institute, Medical Research Centre of Excellence (MRCE), National Research Centre, Cairo, Egypt ,lara26sara@gmail.com
3- Physiology Department, Medical Research and Clinical Studies Institute, Medical Research Centre of Excellence (MRCE), National Research Centre, Cairo, Egypt
2- Department of Child Health, Medical Research and Clinical Studies Institute, Medical Research Centre of Excellence (MRCE), National Research Centre, Cairo, Egypt ,
3- Physiology Department, Medical Research and Clinical Studies Institute, Medical Research Centre of Excellence (MRCE), National Research Centre, Cairo, Egypt
Full-Text [PDF 481 kb]
(932 Downloads)
| Abstract (HTML) (2285 Views)
Full-Text: (696 Views)
Introduction
The predominance of obesity worldwide highlights its impact on increasing the risk of nephropathy in adulthood. Lately, and for the past 30 years, a higher prevalence of renal failure has gone hand in hand with an increase in the prevalence of high body mass index (BMI) (1,2,3). Obesity was also recognized for its powerful influence on acquiring a progressing renal insult. Fortunately, obesity is considered a risk that can be modified (4,5), and astonishingly, recent research has proven a similar phenomenon in childhood obesity. Obesity has potentiated the risks of nephropathy and has remarkably increased the incidence of chronic renal issues (6). The proposed renal pathological pathways in obese subjects are multiple and variable. An obesity-induced renal insult begins with an extra workload on glomerular filtration. The hyperfiltration nephrons are exposed to injurious proteinuria, hypertrophy, and fibrosis (7).
Currently, visceral fat is a well-recognized source of pro-inflammatory, hormonal, and oxidative stressors, which result in insulin resistance and predisposition to numerous metabolic diseases (8). Nowadays, many studies are directed to investigate the role of these stressors in targeting the kidneys and the potential glomerular damage caused by them. The chronic inflammatory milieu induced by obesity may be harmful to renal tissues and may trigger inflammation and fibrosis on a cellular level (9,10). In addition, the co-morbidities in association with obesity are injurious to the kidneys. The mostly encountered ones are systemic hypertension, insulin resistance, hypertriglyceridemia, hypercholesterolemia, sympathetic overactivity, imbalanced leptin/adiponectin hormones and other diseases that may cause a decline in kidney functions (3). The cells of the proximal convoluted tubules are the most sensitive to injury from the previously mentioned conditions (11).
Kidney injury molecule-1 (KIM-1) has been studied in both acutely and chronically injurious renal insults (12,13). KIM-1 is a type 1 cellular membranous glycoprotein (3,14). An elevated level of KIM-1 is always present in cellular damage of the proximal convoluted tubules (15,16). This injury marker has greatly served in assessing renal affection secondary to proteinuria, exposure to toxins, and/or ischemia (17,18).
The chief concern of the current research was to study the sensitivity and specificity of plasma KIM-1 as a screening tool for renal injury in overweight/obese adolescents.
Methods
Ethical approval was granted by the Ethical Committee of the National Research Centre before the start of the study. Written informed consent was signed by one of the parents on the behalf of each child in accordance with the code of ethics of the Declaration of Helsinki.
Ninety children and adolescents with the mean age 13.05 ± 2.61 years were enrolled in the study. The participants were stratified into two groups: Group 1 (Cases) with 45 Egyptian overweight/obese adolescents and Group 2 (Control) with 45 lean Egyptian adolescents. The participants were recruited from the Nutrition and Immunity Clinic at the Medical Research Centre of Excellence, the National Research Centre. The inclusion criteria were both sexes aged 10 to 18, and the exclusion criteria included syndromes of obesity and endocrinal causes of obesity.
Measurements were evaluated for each participant. The height was recorded to the nearest 0.5 cm on a Holtain portable anthropometer, and the weight was registered to the nearest 0.1 kg on a Seca scale balance with minimal clothes and without shoes. BMI was calculated as weight (kg)/height (m2).
Blood samples were collected from all 90 patients and centrifuged. Then, serum was isolated and stored at -20 until collection of all samples. Serum creatinine measurement was performed using a spectrophotometer, and KIM-1 was assessed using ELISA methods (Elabscience Biotechnology Co., Ltd, 1 Shzishanst, Wuhan, Hubei, China, 430070).
Descriptive data were presented as mean and standard deviation. Comparative data between the case and control groups were analyzed, and P-value was calculated. Results were considered significant at a P-value of ≤ 0.05. Statistical analysis was performed using SPSS version 21.
Results
The groups were homogeneous in terms of age with an insignificant P-value of 0.446. As planned, the anthropometric measurements were significantly different between the cases and controls. A highly significant P-value of 0.001 was observed for BMI scores between the cases and controls, as shown in Table 1.
The complete blood count was comparable between the two groups. As shown in Table 2, highly significant P-values of 0.001 and 0.007 were obtained for KIM-1 and creatinine levels, respectively, in the cases compared to the controls.
No correlations were found between KIM-1 and systolic and diastolic blood pressures in the case group, as shown in Table 3.
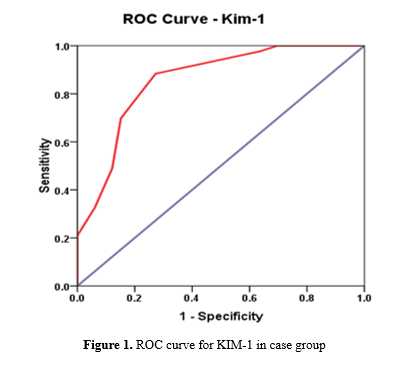
In our study, KIM-1 at a cutoff value of 3.00 was found to be a marker of high significance (P=0.001) for detection of early renal injury with a sensitivity of 88.4% and a specificity of 72.7%, as shown in Table 4 and Figure 1.
Discussion
Adolescence, according to the World Health Organization, is the stage of high vulnerability. Thus, we conducted the current study with special emphasis on this critical stage of life (19-25). To this end, 90 Egyptian adolescents were included in the study. The sex distribution was 58 females (64%) and 32 males (36%). The age ranged between 10-18 years. The participants were grouped according to BMI percentiles: a case group of 45 overweight/obese adolescents and a control group of 45 non-obese adolescents. A high BMI, defined as ≥ 85th percentile, was used as the BMI cutoff point of risk criteria, through which the candidates were stratified into cases and controls. This "BMI-based selection" method was adopted by many authors (26-30). Nineteen out of 32 males (59%) were overweight/obese, compared to 26 out of 58 females (45%) who were overweight/obese.
As a strong point in our research, a highly significant discrepancy was observed in terms of BMI between the cases and controls. The reason is that it pronounced the impact of higher BMI on the obtained results. The P-value was 0.001 between both groups in terms of weight, BMI, and BMI percentiles.
We assessed blood pressure in all participants since hypertension is usually associated with obesity and is believed to impair renal function (31). This association was evident in our cases due to their significantly higher blood pressure compared to the controls. The P-values were 0.000 and 0.002 for measurements of systolic and diastolic pressures, respectively, reflecting the effect of high BMI on hemodynamics. Similarly, studies by Ding et al. and El-Shaheed et al. proved a positive correlation between BMI and both systolic and diastolic pressures among obese adolescents (32,33).
Also, the serum creatinine level was significantly higher in overweight/obese adolescents compared to their non-obese counterparts, with a P-value of 0.007. Although all of our cases had their creatinine levels within the normal range. This finding is in line with the result of a research by Van Dam et al., concluding that obesity negatively affects the creatinine level (34).
In many contemporary studies, KIM-1 has been considered a highly useful biomarker for acute and chronic renal injury (35-37). Urinary KIM-1 is a well-established marker of drug-induced nephrotoxicity, validated by the Food and Drug Administration since 2008 (38). The estimation of the KIM-1 level in blood is a new tool for renal injury assessment investigated in a multitude of recent studies (39,40). To the best of our knowledge, our study is the first to evaluate blood KIM-1 as a sensitive and specific marker of early renal injury in obese adolescents. A significantly higher KIM-1 value was found in the overweight/obese group (P-value=0.001). Blood KIM-1 was proven to have a sensitivity of 88.4% and a specificity of 72.7% at a 95% confidence interval. This is comparable to the findings of Sabbisetti et al., who assessed the sensitivity and specificity of KIM-1 through the ROC curve of blood KIM-1 in kidney injury associated with type 1 diabetes (40).
We did not find significant correlations between KIM-1 and blood pressure measurements in the case group. In contrast, Tomczak et al. found a significant positive correlation between KIM-1 and both systolic and diastolic readings (41).
Therefore, a scheduled routine monitoring of blood pressure measurements and the renal injury marker KIM-1 in overweight/obese children should be considered.
Conclusion
A significantly higher blood pressure was detected in overweight/obese cases compared to controls. A significantly higher KIM-1 value was found in the group of overweight/obese cases, denoting renal affection. With almost 88% sensitivity and 73% specificity, KIM-1 may be considered a complementary method alongside other diagnostic methods for detecting renal injury.
Acknowledgement
The authors acknowledge the children and their caregivers who participated in the study, as well as all co-workers who attempted to improve the work environment for the entire team.
Funding sources
None
Ethical statement
This research was performed in accordance with the code of ethics of the Declaration of Helsinki.
Conflicts of interest
None
Author contributions
Nermine N. conceived the idea, supervised each step in the research, and wrote most of the manuscript. Azza Abd El-Shaheed also contributed to the idea and supervised all phases of the research. Sara F., the corresponding author, collected data and contributed to manuscript writing. Rehab S. was responsible for data collection, resource management, and statistical analysis. Salwa R. and Hiba carried out most of the laboratory work. All authors reviewed and approved the final manuscript.
The predominance of obesity worldwide highlights its impact on increasing the risk of nephropathy in adulthood. Lately, and for the past 30 years, a higher prevalence of renal failure has gone hand in hand with an increase in the prevalence of high body mass index (BMI) (1,2,3). Obesity was also recognized for its powerful influence on acquiring a progressing renal insult. Fortunately, obesity is considered a risk that can be modified (4,5), and astonishingly, recent research has proven a similar phenomenon in childhood obesity. Obesity has potentiated the risks of nephropathy and has remarkably increased the incidence of chronic renal issues (6). The proposed renal pathological pathways in obese subjects are multiple and variable. An obesity-induced renal insult begins with an extra workload on glomerular filtration. The hyperfiltration nephrons are exposed to injurious proteinuria, hypertrophy, and fibrosis (7).
Currently, visceral fat is a well-recognized source of pro-inflammatory, hormonal, and oxidative stressors, which result in insulin resistance and predisposition to numerous metabolic diseases (8). Nowadays, many studies are directed to investigate the role of these stressors in targeting the kidneys and the potential glomerular damage caused by them. The chronic inflammatory milieu induced by obesity may be harmful to renal tissues and may trigger inflammation and fibrosis on a cellular level (9,10). In addition, the co-morbidities in association with obesity are injurious to the kidneys. The mostly encountered ones are systemic hypertension, insulin resistance, hypertriglyceridemia, hypercholesterolemia, sympathetic overactivity, imbalanced leptin/adiponectin hormones and other diseases that may cause a decline in kidney functions (3). The cells of the proximal convoluted tubules are the most sensitive to injury from the previously mentioned conditions (11).
Kidney injury molecule-1 (KIM-1) has been studied in both acutely and chronically injurious renal insults (12,13). KIM-1 is a type 1 cellular membranous glycoprotein (3,14). An elevated level of KIM-1 is always present in cellular damage of the proximal convoluted tubules (15,16). This injury marker has greatly served in assessing renal affection secondary to proteinuria, exposure to toxins, and/or ischemia (17,18).
The chief concern of the current research was to study the sensitivity and specificity of plasma KIM-1 as a screening tool for renal injury in overweight/obese adolescents.
Methods
Ethical approval was granted by the Ethical Committee of the National Research Centre before the start of the study. Written informed consent was signed by one of the parents on the behalf of each child in accordance with the code of ethics of the Declaration of Helsinki.
Ninety children and adolescents with the mean age 13.05 ± 2.61 years were enrolled in the study. The participants were stratified into two groups: Group 1 (Cases) with 45 Egyptian overweight/obese adolescents and Group 2 (Control) with 45 lean Egyptian adolescents. The participants were recruited from the Nutrition and Immunity Clinic at the Medical Research Centre of Excellence, the National Research Centre. The inclusion criteria were both sexes aged 10 to 18, and the exclusion criteria included syndromes of obesity and endocrinal causes of obesity.
Measurements were evaluated for each participant. The height was recorded to the nearest 0.5 cm on a Holtain portable anthropometer, and the weight was registered to the nearest 0.1 kg on a Seca scale balance with minimal clothes and without shoes. BMI was calculated as weight (kg)/height (m2).
Blood samples were collected from all 90 patients and centrifuged. Then, serum was isolated and stored at -20 until collection of all samples. Serum creatinine measurement was performed using a spectrophotometer, and KIM-1 was assessed using ELISA methods (Elabscience Biotechnology Co., Ltd, 1 Shzishanst, Wuhan, Hubei, China, 430070).
Descriptive data were presented as mean and standard deviation. Comparative data between the case and control groups were analyzed, and P-value was calculated. Results were considered significant at a P-value of ≤ 0.05. Statistical analysis was performed using SPSS version 21.
Results
The groups were homogeneous in terms of age with an insignificant P-value of 0.446. As planned, the anthropometric measurements were significantly different between the cases and controls. A highly significant P-value of 0.001 was observed for BMI scores between the cases and controls, as shown in Table 1.
Table 3. Correlations between KIM-1, BMI, and systolic and diastolic blood pressures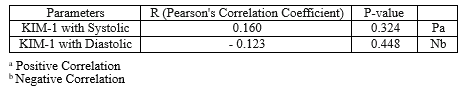 |
|
Table 4. Sensitivity and specificity of KIM-1 in case group
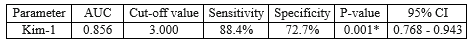 |

Discussion
Adolescence, according to the World Health Organization, is the stage of high vulnerability. Thus, we conducted the current study with special emphasis on this critical stage of life (19-25). To this end, 90 Egyptian adolescents were included in the study. The sex distribution was 58 females (64%) and 32 males (36%). The age ranged between 10-18 years. The participants were grouped according to BMI percentiles: a case group of 45 overweight/obese adolescents and a control group of 45 non-obese adolescents. A high BMI, defined as ≥ 85th percentile, was used as the BMI cutoff point of risk criteria, through which the candidates were stratified into cases and controls. This "BMI-based selection" method was adopted by many authors (26-30). Nineteen out of 32 males (59%) were overweight/obese, compared to 26 out of 58 females (45%) who were overweight/obese.
As a strong point in our research, a highly significant discrepancy was observed in terms of BMI between the cases and controls. The reason is that it pronounced the impact of higher BMI on the obtained results. The P-value was 0.001 between both groups in terms of weight, BMI, and BMI percentiles.
We assessed blood pressure in all participants since hypertension is usually associated with obesity and is believed to impair renal function (31). This association was evident in our cases due to their significantly higher blood pressure compared to the controls. The P-values were 0.000 and 0.002 for measurements of systolic and diastolic pressures, respectively, reflecting the effect of high BMI on hemodynamics. Similarly, studies by Ding et al. and El-Shaheed et al. proved a positive correlation between BMI and both systolic and diastolic pressures among obese adolescents (32,33).
Also, the serum creatinine level was significantly higher in overweight/obese adolescents compared to their non-obese counterparts, with a P-value of 0.007. Although all of our cases had their creatinine levels within the normal range. This finding is in line with the result of a research by Van Dam et al., concluding that obesity negatively affects the creatinine level (34).
In many contemporary studies, KIM-1 has been considered a highly useful biomarker for acute and chronic renal injury (35-37). Urinary KIM-1 is a well-established marker of drug-induced nephrotoxicity, validated by the Food and Drug Administration since 2008 (38). The estimation of the KIM-1 level in blood is a new tool for renal injury assessment investigated in a multitude of recent studies (39,40). To the best of our knowledge, our study is the first to evaluate blood KIM-1 as a sensitive and specific marker of early renal injury in obese adolescents. A significantly higher KIM-1 value was found in the overweight/obese group (P-value=0.001). Blood KIM-1 was proven to have a sensitivity of 88.4% and a specificity of 72.7% at a 95% confidence interval. This is comparable to the findings of Sabbisetti et al., who assessed the sensitivity and specificity of KIM-1 through the ROC curve of blood KIM-1 in kidney injury associated with type 1 diabetes (40).
We did not find significant correlations between KIM-1 and blood pressure measurements in the case group. In contrast, Tomczak et al. found a significant positive correlation between KIM-1 and both systolic and diastolic readings (41).
Therefore, a scheduled routine monitoring of blood pressure measurements and the renal injury marker KIM-1 in overweight/obese children should be considered.
Conclusion
A significantly higher blood pressure was detected in overweight/obese cases compared to controls. A significantly higher KIM-1 value was found in the group of overweight/obese cases, denoting renal affection. With almost 88% sensitivity and 73% specificity, KIM-1 may be considered a complementary method alongside other diagnostic methods for detecting renal injury.
Acknowledgement
The authors acknowledge the children and their caregivers who participated in the study, as well as all co-workers who attempted to improve the work environment for the entire team.
Funding sources
None
Ethical statement
This research was performed in accordance with the code of ethics of the Declaration of Helsinki.
Conflicts of interest
None
Author contributions
Nermine N. conceived the idea, supervised each step in the research, and wrote most of the manuscript. Azza Abd El-Shaheed also contributed to the idea and supervised all phases of the research. Sara F., the corresponding author, collected data and contributed to manuscript writing. Rehab S. was responsible for data collection, resource management, and statistical analysis. Salwa R. and Hiba carried out most of the laboratory work. All authors reviewed and approved the final manuscript.
Article Type: Research |
Subject:
Pediatrics
References
1. Sinha S, Haque M. Insulin Resistance Is Cheerfully Hitched with Hypertension. Life (Basel). 2022;12(4):564. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Wang Q, Zhang JJ, Dou WJ, Zeng HQ, Shi PP, Wu J. Impact of body mass index on primary immunoglobulin A nephropathy prognosis: a systematic review and meta-analysis. Int Urol Nephrol. 2022;54(5):1067-78. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Bielopolski D, Singh N, Bentur OS, Renert-Yuval Y, MacArthur R, Vasquez KS, et al. Obesity Related Glomerulopathy in Adolescent Women: The Effect of Body Surface Area. Kidney360. 2021;3(1):113-21. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Verde L, Lucà S, Cernea S, Sulu C, Yumuk VD, Jenssen TG, et al. The Fat Kidney. Curr Obes Rep. 2023;12(2):86-98 [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Fernández P, Nores ML, Douthat W, de Arteaga J, Luján P, Campazzo M, et al. Estimation of Glomerular Filtration Rate in Obese Patients: Utility of a New Equation. Nutrients. 2023;15(5):1233. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Colasante AM, Bartiromo M, Nardolillo M, Guarino S, Marzuillo P, Mangoni di S Stefano GSRC, et al. Tangled relationship between insulin resistance and microalbuminuria in children with obesity. World J Clin Pediatr. 2022;11(6):455-62. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Aminnejad B, Roumi Z, HasanpourArdekanizadeh N, Vahid F, Gholamalizadeh M, Kalantari N, et al. Association of dietary antioxidant index with body mass index in adolescents. Obes Sci Pract. 2022;9(1):15-22. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Lin CA, Li WC, Lin SY, Chen YC, Yu W, Huang HY, et al. Gender differences in the association between insulin resistance and chronic kidney disease in a Chinese population with metabolic syndrome. Diabetol Metab Syndr. 2022;14(1):184. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Grapin M, Gaillard F, Biebuyck N, Ould-Rabah M, Hennequin C, Berthaud R, et al. The spectrum of kidney function alterations in adolescents with a solitary functioning kidney. Pediatr Nephrol. 2021;36(10):3159-68. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Takeuchi H, Uchida HA, Katayama K, Matsuoka-Uchiyama N, Okamoto S, Onishi Y, et al. The Beneficial Effect of Personalized Lifestyle Intervention in Chronic Kidney Disease Follow-Up Project for National Health Insurance Specific Health Checkup: A Five-Year Community-Based Cohort Study. Medicina (Kaunas). 2022;58(11):1529. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Mangat G, Nair N, Barat O, Abboud B, Pais P, Bagga S, et al. Obesity-related glomerulopathy in children: connecting pathophysiology to clinical care. Clin Kidney J. 2022;16(4):611-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Huang R, Fu P, Ma L. Kidney fibrosis: from mechanisms to therapeutic medicines. Signal Transduct Target Ther. 2023;8(1):129. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Pál K, Mănescu IB, Lupu S, Dobreanu M. Emerging Biomarkers for Predicting Clinical Outcomes in Patients with Heart Disease. Life (Basel). 2023;13(1):230. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Uribe-Restrepo P, Munoz-Zanzi C, Agudelo-Flórez P. Kidney Injury Biomarkers in Leptospirosis. Rev Soc Bras Med Trop. 2023;56:e0260-2022. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Venkatesan A, Roy A, Kulandaivel S, Natesan V, Kim SJ. p-Coumaric Acid Nanoparticles Ameliorate Diabetic Nephropathy via Regulating mRNA Expression of KIM-1 and GLUT-2 in Streptozotocin-Induced Diabetic Rats. Metabolites. 2022;12(12):1166. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Mahapatra HS, Kulshreshtha B, Goyal P, Chitkara A, Kumari A, Arora A, et al. Comparative diagnostic utility of different urinary biomarkers during pre-albuminuric stages of non-hypertensive type 2 diabetic nephropathy. Indian J Med Res. 2022;156(1):46-55. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Bartos K, Moor MB. FGFR regulator Memo1 is dispensable for FGF23 expression by osteoblasts during folic acid-driven kidney injury. Physiol Rep. 2023;11(6):e15650. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. An HS, Yoo JW, Jeong JH, Heo M, Hwang SH, Jang HM, et al. Lipocalin-2 promotes acute lung inflammation and oxidative stress by enhancing macrophage iron accumulation. Int J Biol Sci. 2023;19(4):1163-77. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Al Makadma AS. Review article: Adolescent health and health care in the Arab Gulf countries: Today's needs and tomorrow's challenges. Int J Pediatr Adolesc Med. 2017;4(1):1-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. WHO. Adolescent health and development. 2019. [View at Publisher]
21. Raouf NR, Mahfouz EM,Seedhom AE, Ghazawy ER, Abdelrehim MG. The risk of Obesity in relation to dietary habits among medical students at Minia University: Faculty of medicine, Minia, Egypt. Minia Journal of Medical Research. 2022;33(4):105-14. [View at Publisher] [DOI] [Google Scholar]
22. Musaiger AO, Nabag FO, and Al-Mannai M. Obesity, Dietary Habits, and Sedentary Behaviors Among Adolescents in Sudan: Alarming Risk Factors for Chronic Diseases in a Poor Country. Food and Nutrition Bulletin. 2016;37(1):65-72. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Liu K, Zhang J, Liu S, Chen J, Zhang Y, Li W, et al. Parental Stress on Children's Appearance, Body Dissatisfaction, and Eating Behaviours in Chinese Children: A Pathway Analysis. Psychol Res Behav Manag. 2023;16:363-72. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Bhattacharjee P, Mukhopadhyay S, Joshi P, Singh S. Food habits and obesity: a study in adolescents. Int J ContempPediatr. 2017;4(2):336-340 [View at Publisher] [DOI] [Google Scholar]
25. Zeidan W, Taweel H, Shalash A, Husseini A. Consumption of fruits and vegetables among adolescents in Arab Countries: a systematic review. Int J Behav Nutr Phys Act. 2023;20(1):3. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Ibrahim OM, Gabre AA, Sallam SF, El-Alameey IR, Sabry RN, Galal EM, et al. Influence of Interleukin-6 (174G/C) Gene Polymorphism on Obesity in Egyptian Children. Open Access Maced J Med Sci. 2017;5(7):831-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. El Wakeel MA, El-Kassas GM, Kamhawy AH, Galal EM, Nassar MS, Hammad EM, et al. Serum Apelin and Obesity-Related Complications in Egyptian Children. Open Access Maced J Med Sci. 2018;6(8):1354-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. El Kassas GM, Shehata MA, El Wakeel MA, Amer AF, Elzaree FA, Darwish MK, et al. Role of Procalcitonin As an Inflammatory Marker in a Sample of Egyptian Children with Simple Obesity. Open Access Maced J Med Sci. 2018;6(8):1349-53. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Mahfouz NN, Fahmy RF, Nassar MS, Wahba SA. Body Weight Concern and Belief among Adolescent Egyptian Girls. Open Access Maced J Med Sci. 2018;6:(3):582-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Sorkhi H, Haji Aahmadi M. Urinary Calcium to Creatinine Ratio in Children. Indian J Pediatr. 2005;72(12):1055-6 [View at Publisher] [DOI] [PMID] [Google Scholar]
31. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(Supple_2):555-76. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Ding W, Cheung WW, Mak RH. Impact of obesity on kidney function and blood pressure in children. World J Nephrol. 2015;4(2):223-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
33. El-Shaheed AA, Moustafa RS, Sallam SF, Mahfouz NN, El-Zayat SR, Sibaii H, et al. Estimated glomerular filtration rate and blood pressure in a sample of obese Egyptian adolescents. J Arab Soc Med Res. 2022;17(1):89-95. [View at Publisher] [DOI] [Google Scholar]
34. Van Dam MJCM, Pottel H, Vreugdenhil ACE. Relation between obesity-related comorbidities and kidney function estimation in children. Pediatr Nephrol. 2023;38(6):1867-76. [View at Publisher] [DOI] [PMID] [Google Scholar]
35. Köksoy AY, Görükmez Ö, Dorum S. Clinical significance of hypouricemia in children and adolescents. Pediatr Nephrol. 2023;38(9):3017-25. [View at Publisher] [DOI] [PMID] [Google Scholar]
36. Antonio-Villa NE, Juárez-Rojas JG, Posadas-Sánchez R, Reyes-Barrera J, Medina-Urrutia A. Visceral adipose tissue is an independent predictor and mediator of the progression of coronary calcification: a prospective sub-analysis of the GEA study. Cardiovasc Diabetol. 2023;22(1):81. [View at Publisher] [DOI] [PMID] [Google Scholar]
37. Valerio G, Licenziati MR, Iannuzzi A, Franseze A, Siani P, Riccardi G, et al. Insulin resistance and impaired glucose tolerance in obese children and adolescents from southern Italy. Nutr Metab Cardiovasc Dis. 2006;16(4):279-84 [View at Publisher] [DOI] [PMID] [Google Scholar]
38. Chen R, Sanyal S, Thompson A, Ix JH, Haskins K, Muldowney L, et al. Evaluating the Use of KIM-1 in Drug Development and Research Following FDA Qualification. Clin Pharmacol Ther. 2018;104(6):1175-81. [View at Publisher] [DOI] [PMID] [Google Scholar]
39. Huang J, CaliskanGuzelce E, Gholami SK, Gawelek KL, Mitchell RN, Pojoga LH, et al. Effects of Mineralocorticoid Receptor Blockade and Statins on Kidney Injury Marker 1 (KIM-1) in Female Rats Receiving L-NAME and Angiotensin II. Int J Mol Sci. 2023;24(7):6500. [View at Publisher] [DOI] [PMID] [Google Scholar]
40. Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25(10):2177-86. [View at Publisher] [DOI] [PMID] [Google Scholar]
41. Tomczak J, Wasilewska A, Milewski R. Urine NGAL and KIM-1 in children and adolescents with hyperuricemia. Pediatr Nephrol. 2013;28(9):1863-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





