Volume 8, Issue 4 (Journal of Clinical and Basic Research (JCBR) 2024)
jcbr 2024, 8(4): 1-4 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Adebo D O, Olaniyan M F, Daramola G O, Ugege C O, Odegbemi O B. Association of PPARG genotypes with biochemical markers in type 2 diabetes mellitus among a Nigerian population. jcbr 2024; 8 (4) :1-4
URL: http://jcbr.goums.ac.ir/article-1-464-en.html
URL: http://jcbr.goums.ac.ir/article-1-464-en.html
David Olufemi Adebo1 

 , Mathew Folaranmi Olaniyan2
, Mathew Folaranmi Olaniyan2 

 , Gabriel Olufemi Daramola3
, Gabriel Olufemi Daramola3 
 , Christian Onosetale Ugege2
, Christian Onosetale Ugege2 

 , Odekunle Bola Odegbemi *4
, Odekunle Bola Odegbemi *4 




 , Mathew Folaranmi Olaniyan2
, Mathew Folaranmi Olaniyan2 

 , Gabriel Olufemi Daramola3
, Gabriel Olufemi Daramola3 
 , Christian Onosetale Ugege2
, Christian Onosetale Ugege2 

 , Odekunle Bola Odegbemi *4
, Odekunle Bola Odegbemi *4 


1- Medical Laboratory Science Department, Edo State University, Uzairue, Edo State, Nigeria ; Chemical Pathology Department, Ekiti State University Teaching Hospital, Ado-Ekiti, Ekiti State, Nigeria
2- Medical Laboratory Science Department, Edo State University, Uzairue, Edo State, Nigeria
3- Medical Microbiology and Parasitology Department, Ekiti State University Teaching Hospital, Ado-Ekiti, Ekiti State, Nigeria ; Medical Laboratory Science Department, College of Medicine, Ekiti State University, Ado-Ekiti, Ekiti State, Nigeria
4- Medical Laboratory Science Department, Edo State University, Uzairue, Edo State, Nigeria;Medical Laboratory Science Department, Nigerian Navy Hospital, Warri, Delta State, Nigeria ,odegbemi21.odekunle@edouniversity.edu.ng
2- Medical Laboratory Science Department, Edo State University, Uzairue, Edo State, Nigeria
3- Medical Microbiology and Parasitology Department, Ekiti State University Teaching Hospital, Ado-Ekiti, Ekiti State, Nigeria ; Medical Laboratory Science Department, College of Medicine, Ekiti State University, Ado-Ekiti, Ekiti State, Nigeria
4- Medical Laboratory Science Department, Edo State University, Uzairue, Edo State, Nigeria;Medical Laboratory Science Department, Nigerian Navy Hospital, Warri, Delta State, Nigeria ,
Full-Text [PDF 499 kb]
(763 Downloads)
| Abstract (HTML) (2423 Views)
Discussion
The study highlights the role of the PPARG gene in metabolic regulation, suggesting that tailoring diabetes management based on genotype could help mitigate specific risks, such as renal or hepatic complications, thereby enhancing treatment efficacy for Nigerian diabetics. The demographic characteristics of the study population reveal a significant prevalence of individuals over 50 years old, consistent with the typical age of onset for T2DM, which increases significantly after age 45 (11). The higher percentage of the female participants (70-75%) may reflect gender-specific health-seeking behaviors or a higher prevalence of T2DM among women in this population, despite global trends showing a slightly higher prevalence in men (12).
Findings from this study on PPARG genotypes in the Nigerian diabetic population provide significant insights into the relationship between genetic factors and organ health markers, particularly Cystatin C and ALT. Elevated levels of Cystatin C indicate early renal dysfunction, a common complication associated with diabetes, while increased ALT levels suggest potential liver involvement. The association of the GG genotype with poorer biochemical outcomes emphasizes the importance of considering genetic factors in assessing the risk of T2DM and in treatment planning (13-18).
The study found that the diabetic subjects exhibited elevated levels of Cystatin C (1.46 mg/L vs. 0.98 mg/L in controls, p=0.001) and ALT (71.85 U/L vs. 27.51 U/L, p=0.001), indicating potential organ dysfunction. The presence of the GG genotype was more frequent in the diabetic group, aligning with previous research linking PPARG polymorphisms to T2DM risk. Lower levels of IL-10 in the diabetic females suggest chronic low-grade inflammation, which is often associated with diabetes. The elevated CK-MB levels in the diabetic females indicate potential subclinical cardiac damage, supporting findings that diabetes increases the risk of cardiovascular complications (19).
The observed reduction in IL-10 levels and elevation in other inflammatory markers among the diabetic participants may indicate a hyperglycemia-induced inflammatory response, consistent with prior studies demonstrating that acute hyperglycemia can increase inflammatory cytokines due to oxidative stress mechanisms (20).
The study's modest sample size and ethnic specificity may limit the generalizability of the results. Additionally, the cross-sectional design restricts the ability to establish causality in the observed associations. The associations between PPARG genotypes and various biochemical markers provide new insights into the mechanisms by which PPARG might influence diabetes pathophysiology. For instance, higher levels of Cystatin C in the GS genotype may indicate a link between PPARG and renal function, consistent with studies showing that PPARG agonists can improve renal outcomes in diabetic patients (21). Elevated ALT levels in the SS genotype could suggest a relationship between PPARG and hepatic function, aligning with research on PPARG's role in hepatic lipid metabolism and insulin sensitivity (22).
Moreover, higher levels of IL-10 in the GG genotype suggest a potential anti-inflammatory effect, supported by evidence that PPARG activation can modulate inflammatory responses (23). Finally, higher fasting blood sugar levels in the SS genotype indicate a potential association with poorer glycemic control, consistent with studies linking specific PPARG polymorphisms to increased diabetes risk and altered insulin sensitivity (24). These findings underscore the complex interplay between PPARG genotypes, diabetes, and various organ systems, suggesting that PPARG may influence diabetes pathophysiology through multiple mechanisms. However, further research, including functional studies and larger cohorts, is necessary to fully understand these relationships and their clinical implications. The study's modest sample size and ethnic specificity may limit the generalizability of the results. Additionally, the cross-sectional design restricts the ability to establish causality in the observed associations.
Conclusion
This study underscores the complexity of T2DM pathophysiology and the importance of considering genetic and biochemical factors in diabetes research and clinical practice. These findings may pave the way for more targeted interventions and personalized medicine approaches in managing T2DM.
This study provides a detailed analysis of the relationship between PPARG genotypes and T2DM in a Nigerian population, highlighting critical demographic and biochemical characteristics (11-14). Key findings include the higher prevalence of the GG genotype among diabetic individuals and significant associations between PPARG genotypes and markers of organ function, such as elevated Cystatin C and ALT, which suggest early renal and hepatic involvement. Furthermore, lower IL-10 levels among diabetic patients may indicate a genetic influence on inflammatory processes in T2DM (17-24).
These insights enhance our understanding of how PPARG genotypes contribute to T2DM susceptibility and complications in this population, underscoring the potential for incorporating genetic markers into personalized diabetes management (15). Further research with larger and more diverse cohorts, as well as functional analyses, is needed to validate these findings and explore the mechanisms by which PPARG genotypes affect diabetes pathophysiology (16). Such studies could ultimately support targeted prevention strategies and tailored treatment approaches for T2DM patients based on genetic profiles.
Acknowledgement
The management and staff of the Chemical Pathology Department, Ekiti State University Teaching Hospital, Ado-Ekiti, Ekiti State, Nigeria, are appreciated for providing the enabling environment for conducting this study.
Funding sources
There was no external funding for this research.
Ethical statement
We obtained ethical clearance for this study from the Ethics and Research Committee of the University Teaching Hospital (Protocol Number: EKSUTH/A67/2022/12/022). Additionally, the research adhered to ethical principles, including:
i. Informed Consent: Each participant received a written informed consent form along with the questionnaire, ensuring their consent to participate.
ii. Data Confidentiality: Findings from the study were kept confidential and shared only among co-investigators.
iii. Beneficence: The findings were provided to the managing clinical team at no charge.
iv. Voluntariness: All subjects had the option to decline participation in the study when approached.
Conflicts of interest
The authors declare that there are no conflicts of interest to disclose.
Author contributions
DOA participated in research design, sample collection/analysis, and manuscript writing. MFO participated in research design and overall research supervision. GOD participated in data collation and manuscript writing. COU participated in sample collection and sample analysis. OBO participated in research design, data analysis, and manuscript writing.
Full-Text: (467 Views)
Introduction
Diabetes mellitus is a chronic condition that is increasingly prevalent worldwide, particularly in developing countries like Nigeria (1,2). Currently, the estimated prevalence of diabetes in Nigeria stands at 5.7%, posing significant health challenges for the population. This is particularly concerning given the high rates of complications associated with diabetes, including nephropathy, neuropathy, and cardiovascular disease, which can severely impact the quality of life and increase healthcare costs (3-7).
Type 2 diabetes mellitus (T2DM) arises from a complex interplay of genetic and environmental factors. Among the genetic influences, the peroxisome proliferator-activated receptor gamma (PPARG) gene plays a crucial role in regulating glucose and lipid metabolism (8,9). Specific variants of this gene have been linked to increased susceptibility to T2DM and its complications. However, research focusing on the PPARG gene within African populations remains limited, highlighting a gap in understanding its significance in the context of T2DM in Nigeria (10).
In Ekiti State, the burden of diabetes presents a notable public health challenge, yet data regarding genetic predispositions, particularly concerning PPARG variants, are scarce. Identifying these genetic factors is essential for developing personalized strategies for diabetes prevention and treatment (10). This study aimed to investigate the role of PPARG variants in Nigerian T2DM patients and their possible associations with diabetes-related complications. By enhancing our understanding of these genetic influences, we hope to contribute valuable insights that could lead to improved and personalized diabetes care tailored to the unique genetic landscape of the Nigerian population.
Methods
Study area and population
This study was a cross-sectional study with a control arm that examined the relationship between PPARG genotypes and biochemical markers among diabetic and non-diabetic individuals in a Nigerian population. The study was conducted in Ado-Ekiti, the capital city of Ekiti State, Nigeria (7°40'N, 5°16'E). Ado-Ekiti, with a population of 308,321 (2006 census), is characterized by undulating terrain and prominent inselbergs. The study population comprised patients attending the endocrinology clinic of a hospital.
Sample size calculation
The sample size was determined based on the prevalence rate of the study and calculated using the formula recommended by Uzoagulu (1998). The sample size was calculated based on the expected effect size, a power level of 80%, and a significance level of 0.05.

where N = the number of patients to be sampled
Zα = the standard normal deviation corresponding to a 95% confidence level = 1.96
P = prevalence (5.6% from Elegbede et al., 2022)
d = the degree of accuracy desired (2% = 0.02).
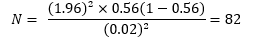
The calculated sample size was 82. With a 15% attrition rate (12.3), the total minimum sample size was approximately 94 subjects.
Sample size justification and sampling
The study included 74 diabetic patients and 20 non-diabetic controls, a sample size chosen to provide preliminary insights into genetic and biochemical associations in this population. Age and gender matching were performed to minimize confounding. While relatively modest, this sample size allows for identifying significant trends that could inform larger future studies.
Inclusion and exclusion criteria
The inclusion criteria encompassed male and female diabetic patients aged 18-65 years. Subjects who tested positive for malaria and chronic diseases such as HIV, tuberculosis, and hepatitis B virus were excluded to minimize confounding factors.
Data collection
We collected demographic and socioeconomic data using a self-administered questionnaire and whole blood samples from all consenting subjects via venipuncture.
Biochemical analysis
We assayed the subjects' FBS, AST, ALT, CK-MB, total protein, Cystatin C, urea, creatinine, and IL-10 levels, and then analyzed the subjects' samples molecularly. FBS, AST, ALT, CK-MB, total protein, urea, creatinine, and Cystatin C levels were measured using the colorimetric-spectrophotometric method, while the IL-10 level was assessed using the ELISA method.
Cystatin C, ALT, CK-MB, and IL-10 were selected based on their relevance to diabetes complications. Cystatin C is a sensitive marker of renal function, often elevated in early diabetic nephropathy. ALT serves as an indicator of liver health, pertinent given the association of diabetes with non-alcoholic fatty liver disease. CK-MB is a cardiac marker, relevant due to the increased cardiovascular risk in diabetics, and IL-10 is an anti-inflammatory cytokine, representing the role of chronic inflammation in T2DM.
Molecular analysis
Genotyping of the CAPN10 (rs384257) polymorphism was performed using PCR amplification. The reaction mixture (25 μL) contained 10 μL genomic DNA, 21 μL S4 fidelity PCR (Dye) Master Mix, and 1 μL each of forward and reverse primers (10 mmol). PCR conditions were as follows: initial denaturation at 98°C for 2 min, followed by 35 cycles of 98°C for 10 s, 59°C for 15 s, and 72°C for 30 s, with a final extension at 72°C for 5 min. PCR products were visualized on a 3% agarose gel stained with SYBR Safe under UV illumination.
Statistical analysis
Data were analyzed using SPSS Version 23.0 (Chicago, USA). Chi-square tests were used for categorical variables, while t-tests or ANOVA were used for continuous variables, with statistical significance set at p < 0.05.
Results
Table 1 indicates that approximately 70% of participants in both the case and control groups were over 50 years old, with average ages of 52 and 50, respectively. The gender distribution was similar, with women comprising 70-75% of each group, and there were no significant differences in age or sex. Figure 1 shows the distribution of three PPARG genotypes (GG, GS, and SS), with the case group exhibiting higher frequencies for the GG (44 vs. 11), GS (18 vs. 5), and SS (12 vs. 4) genotypes compared to the control group, suggesting an association with diabetes risk.
Table 2 compares hepatic, renal, and cardiac markers between the diabetic subjects and the healthy controls, while Tables 3 and 4 disaggregate results by gender. Among the diabetic patients, the prevalence of the GG genotype was higher (59.5%) compared to the controls (55%). The diabetic subjects showed elevated levels of Cystatin C (1.46±0.48 mg/L vs. 0.98±0.33 mg/L, p=0.001), ALT (71.85±4.07 U/L vs. 27.51±3.28 U/L, p=0.001), and CK-MB (11.84±2.79 ng/mL vs. 9.73±2.44 ng/mL, p=0.003). The female diabetic subjects had significantly lower IL-10 levels compared to their male counterparts (88.08±46.99 pg/mL vs. 176.14±28.05 pg/mL, p=0.001).
As shown in Table 5, significant variations were observed in the biochemical markers across different PPARG genotypes. For example, the GG genotype was associated with higher Cystatin C levels (1.36±0.26 ng/mL, p=0.002) and ALT (29.23±4.36 U/L, p=0.030), suggesting potential organ-specific impacts of this genotype on renal and hepatic functions.
Diabetes mellitus is a chronic condition that is increasingly prevalent worldwide, particularly in developing countries like Nigeria (1,2). Currently, the estimated prevalence of diabetes in Nigeria stands at 5.7%, posing significant health challenges for the population. This is particularly concerning given the high rates of complications associated with diabetes, including nephropathy, neuropathy, and cardiovascular disease, which can severely impact the quality of life and increase healthcare costs (3-7).
Type 2 diabetes mellitus (T2DM) arises from a complex interplay of genetic and environmental factors. Among the genetic influences, the peroxisome proliferator-activated receptor gamma (PPARG) gene plays a crucial role in regulating glucose and lipid metabolism (8,9). Specific variants of this gene have been linked to increased susceptibility to T2DM and its complications. However, research focusing on the PPARG gene within African populations remains limited, highlighting a gap in understanding its significance in the context of T2DM in Nigeria (10).
In Ekiti State, the burden of diabetes presents a notable public health challenge, yet data regarding genetic predispositions, particularly concerning PPARG variants, are scarce. Identifying these genetic factors is essential for developing personalized strategies for diabetes prevention and treatment (10). This study aimed to investigate the role of PPARG variants in Nigerian T2DM patients and their possible associations with diabetes-related complications. By enhancing our understanding of these genetic influences, we hope to contribute valuable insights that could lead to improved and personalized diabetes care tailored to the unique genetic landscape of the Nigerian population.
Methods
Study area and population
This study was a cross-sectional study with a control arm that examined the relationship between PPARG genotypes and biochemical markers among diabetic and non-diabetic individuals in a Nigerian population. The study was conducted in Ado-Ekiti, the capital city of Ekiti State, Nigeria (7°40'N, 5°16'E). Ado-Ekiti, with a population of 308,321 (2006 census), is characterized by undulating terrain and prominent inselbergs. The study population comprised patients attending the endocrinology clinic of a hospital.
Sample size calculation
The sample size was determined based on the prevalence rate of the study and calculated using the formula recommended by Uzoagulu (1998). The sample size was calculated based on the expected effect size, a power level of 80%, and a significance level of 0.05.

where N = the number of patients to be sampled
Zα = the standard normal deviation corresponding to a 95% confidence level = 1.96
P = prevalence (5.6% from Elegbede et al., 2022)
d = the degree of accuracy desired (2% = 0.02).
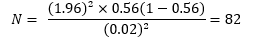
The calculated sample size was 82. With a 15% attrition rate (12.3), the total minimum sample size was approximately 94 subjects.
Sample size justification and sampling
The study included 74 diabetic patients and 20 non-diabetic controls, a sample size chosen to provide preliminary insights into genetic and biochemical associations in this population. Age and gender matching were performed to minimize confounding. While relatively modest, this sample size allows for identifying significant trends that could inform larger future studies.
Inclusion and exclusion criteria
The inclusion criteria encompassed male and female diabetic patients aged 18-65 years. Subjects who tested positive for malaria and chronic diseases such as HIV, tuberculosis, and hepatitis B virus were excluded to minimize confounding factors.
Data collection
We collected demographic and socioeconomic data using a self-administered questionnaire and whole blood samples from all consenting subjects via venipuncture.
Biochemical analysis
We assayed the subjects' FBS, AST, ALT, CK-MB, total protein, Cystatin C, urea, creatinine, and IL-10 levels, and then analyzed the subjects' samples molecularly. FBS, AST, ALT, CK-MB, total protein, urea, creatinine, and Cystatin C levels were measured using the colorimetric-spectrophotometric method, while the IL-10 level was assessed using the ELISA method.
Cystatin C, ALT, CK-MB, and IL-10 were selected based on their relevance to diabetes complications. Cystatin C is a sensitive marker of renal function, often elevated in early diabetic nephropathy. ALT serves as an indicator of liver health, pertinent given the association of diabetes with non-alcoholic fatty liver disease. CK-MB is a cardiac marker, relevant due to the increased cardiovascular risk in diabetics, and IL-10 is an anti-inflammatory cytokine, representing the role of chronic inflammation in T2DM.
Molecular analysis
Genotyping of the CAPN10 (rs384257) polymorphism was performed using PCR amplification. The reaction mixture (25 μL) contained 10 μL genomic DNA, 21 μL S4 fidelity PCR (Dye) Master Mix, and 1 μL each of forward and reverse primers (10 mmol). PCR conditions were as follows: initial denaturation at 98°C for 2 min, followed by 35 cycles of 98°C for 10 s, 59°C for 15 s, and 72°C for 30 s, with a final extension at 72°C for 5 min. PCR products were visualized on a 3% agarose gel stained with SYBR Safe under UV illumination.
Statistical analysis
Data were analyzed using SPSS Version 23.0 (Chicago, USA). Chi-square tests were used for categorical variables, while t-tests or ANOVA were used for continuous variables, with statistical significance set at p < 0.05.
Results
Table 1 indicates that approximately 70% of participants in both the case and control groups were over 50 years old, with average ages of 52 and 50, respectively. The gender distribution was similar, with women comprising 70-75% of each group, and there were no significant differences in age or sex. Figure 1 shows the distribution of three PPARG genotypes (GG, GS, and SS), with the case group exhibiting higher frequencies for the GG (44 vs. 11), GS (18 vs. 5), and SS (12 vs. 4) genotypes compared to the control group, suggesting an association with diabetes risk.
Table 2 compares hepatic, renal, and cardiac markers between the diabetic subjects and the healthy controls, while Tables 3 and 4 disaggregate results by gender. Among the diabetic patients, the prevalence of the GG genotype was higher (59.5%) compared to the controls (55%). The diabetic subjects showed elevated levels of Cystatin C (1.46±0.48 mg/L vs. 0.98±0.33 mg/L, p=0.001), ALT (71.85±4.07 U/L vs. 27.51±3.28 U/L, p=0.001), and CK-MB (11.84±2.79 ng/mL vs. 9.73±2.44 ng/mL, p=0.003). The female diabetic subjects had significantly lower IL-10 levels compared to their male counterparts (88.08±46.99 pg/mL vs. 176.14±28.05 pg/mL, p=0.001).
As shown in Table 5, significant variations were observed in the biochemical markers across different PPARG genotypes. For example, the GG genotype was associated with higher Cystatin C levels (1.36±0.26 ng/mL, p=0.002) and ALT (29.23±4.36 U/L, p=0.030), suggesting potential organ-specific impacts of this genotype on renal and hepatic functions.
Table 1. Sociodemographic data of subjects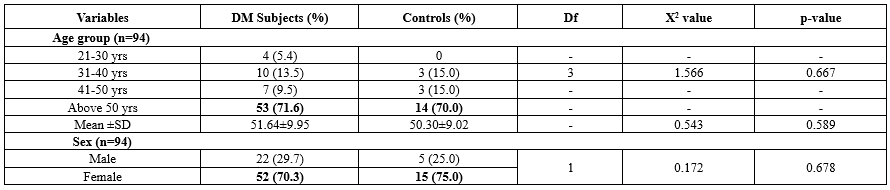 Figure 1. Distribution of PPARG genotypes among diabetic and non-diabetic participants  Table 2. Comparison of biochemical markers between diabetic and non-diabetic participants  |
|
Table 3. Comparison of biochemical markers between diabetic and non-diabetic male participants
 Table 4. Comparison of biochemical markers between diabetic and non-diabetic female participants  Table 5. Comparative analysis of average variables across genotype categories among diabetic subjects in Ekiti state  |
Discussion
The study highlights the role of the PPARG gene in metabolic regulation, suggesting that tailoring diabetes management based on genotype could help mitigate specific risks, such as renal or hepatic complications, thereby enhancing treatment efficacy for Nigerian diabetics. The demographic characteristics of the study population reveal a significant prevalence of individuals over 50 years old, consistent with the typical age of onset for T2DM, which increases significantly after age 45 (11). The higher percentage of the female participants (70-75%) may reflect gender-specific health-seeking behaviors or a higher prevalence of T2DM among women in this population, despite global trends showing a slightly higher prevalence in men (12).
Findings from this study on PPARG genotypes in the Nigerian diabetic population provide significant insights into the relationship between genetic factors and organ health markers, particularly Cystatin C and ALT. Elevated levels of Cystatin C indicate early renal dysfunction, a common complication associated with diabetes, while increased ALT levels suggest potential liver involvement. The association of the GG genotype with poorer biochemical outcomes emphasizes the importance of considering genetic factors in assessing the risk of T2DM and in treatment planning (13-18).
The study found that the diabetic subjects exhibited elevated levels of Cystatin C (1.46 mg/L vs. 0.98 mg/L in controls, p=0.001) and ALT (71.85 U/L vs. 27.51 U/L, p=0.001), indicating potential organ dysfunction. The presence of the GG genotype was more frequent in the diabetic group, aligning with previous research linking PPARG polymorphisms to T2DM risk. Lower levels of IL-10 in the diabetic females suggest chronic low-grade inflammation, which is often associated with diabetes. The elevated CK-MB levels in the diabetic females indicate potential subclinical cardiac damage, supporting findings that diabetes increases the risk of cardiovascular complications (19).
The observed reduction in IL-10 levels and elevation in other inflammatory markers among the diabetic participants may indicate a hyperglycemia-induced inflammatory response, consistent with prior studies demonstrating that acute hyperglycemia can increase inflammatory cytokines due to oxidative stress mechanisms (20).
The study's modest sample size and ethnic specificity may limit the generalizability of the results. Additionally, the cross-sectional design restricts the ability to establish causality in the observed associations. The associations between PPARG genotypes and various biochemical markers provide new insights into the mechanisms by which PPARG might influence diabetes pathophysiology. For instance, higher levels of Cystatin C in the GS genotype may indicate a link between PPARG and renal function, consistent with studies showing that PPARG agonists can improve renal outcomes in diabetic patients (21). Elevated ALT levels in the SS genotype could suggest a relationship between PPARG and hepatic function, aligning with research on PPARG's role in hepatic lipid metabolism and insulin sensitivity (22).
Moreover, higher levels of IL-10 in the GG genotype suggest a potential anti-inflammatory effect, supported by evidence that PPARG activation can modulate inflammatory responses (23). Finally, higher fasting blood sugar levels in the SS genotype indicate a potential association with poorer glycemic control, consistent with studies linking specific PPARG polymorphisms to increased diabetes risk and altered insulin sensitivity (24). These findings underscore the complex interplay between PPARG genotypes, diabetes, and various organ systems, suggesting that PPARG may influence diabetes pathophysiology through multiple mechanisms. However, further research, including functional studies and larger cohorts, is necessary to fully understand these relationships and their clinical implications. The study's modest sample size and ethnic specificity may limit the generalizability of the results. Additionally, the cross-sectional design restricts the ability to establish causality in the observed associations.
Conclusion
This study underscores the complexity of T2DM pathophysiology and the importance of considering genetic and biochemical factors in diabetes research and clinical practice. These findings may pave the way for more targeted interventions and personalized medicine approaches in managing T2DM.
This study provides a detailed analysis of the relationship between PPARG genotypes and T2DM in a Nigerian population, highlighting critical demographic and biochemical characteristics (11-14). Key findings include the higher prevalence of the GG genotype among diabetic individuals and significant associations between PPARG genotypes and markers of organ function, such as elevated Cystatin C and ALT, which suggest early renal and hepatic involvement. Furthermore, lower IL-10 levels among diabetic patients may indicate a genetic influence on inflammatory processes in T2DM (17-24).
These insights enhance our understanding of how PPARG genotypes contribute to T2DM susceptibility and complications in this population, underscoring the potential for incorporating genetic markers into personalized diabetes management (15). Further research with larger and more diverse cohorts, as well as functional analyses, is needed to validate these findings and explore the mechanisms by which PPARG genotypes affect diabetes pathophysiology (16). Such studies could ultimately support targeted prevention strategies and tailored treatment approaches for T2DM patients based on genetic profiles.
Acknowledgement
The management and staff of the Chemical Pathology Department, Ekiti State University Teaching Hospital, Ado-Ekiti, Ekiti State, Nigeria, are appreciated for providing the enabling environment for conducting this study.
Funding sources
There was no external funding for this research.
Ethical statement
We obtained ethical clearance for this study from the Ethics and Research Committee of the University Teaching Hospital (Protocol Number: EKSUTH/A67/2022/12/022). Additionally, the research adhered to ethical principles, including:
i. Informed Consent: Each participant received a written informed consent form along with the questionnaire, ensuring their consent to participate.
ii. Data Confidentiality: Findings from the study were kept confidential and shared only among co-investigators.
iii. Beneficence: The findings were provided to the managing clinical team at no charge.
iv. Voluntariness: All subjects had the option to decline participation in the study when approached.
Conflicts of interest
The authors declare that there are no conflicts of interest to disclose.
Author contributions
DOA participated in research design, sample collection/analysis, and manuscript writing. MFO participated in research design and overall research supervision. GOD participated in data collation and manuscript writing. COU participated in sample collection and sample analysis. OBO participated in research design, data analysis, and manuscript writing.
Article Type: Research |
Subject:
Biochemistry
References
1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Supplement 1):S81-90. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. 2006. [View at Publisher] [Google Scholar]
3. International Diabetes Federation. IDF Diabetes Atlas, 9th Ed. Brussels, Belgium:International Diabetes Federation;2019. [View at Publisher] [Google Scholar]
4. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4-14. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328(23):1676-85. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26(2):77-82. [View at Publisher] [DOI] [Google Scholar]
7. Uloko AE, Musa BM, Ramalan MA, Gezawa ID, Puepet FH, Uloko AT, et al. Prevalence and risk factors for diabetes mellitus in Nigeria: a systematic review and meta-analysis. Diabetes Ther. 2018;9(3):1307-16. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Ogbera AO, Ekpebegh C. Diabetes mellitus in Nigeria: The past, present and future. World J Diabetes. 2014;5(6):905-11. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Lima JEBF, Moreira NCS, Sakamoto-Hojo ET. Mechanisms underlying the pathophysiology of type 2 diabetes: From risk factors to oxidative stress, metabolic dysfunction, and hyperglycemia. Mutat Res Genet Toxicol Environ Mutagen. 2022;874-875:503437. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, et al. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402(6764):880-3. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. American Diabetes Association. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15-33. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Stumvoll M, Häring H. The peroxisome proliferator-activated receptor-gamma2 Pro12 Ala polymorphism. Diabetes. 2002;51(8):2341-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Gouda HN, Sagoo GS, Harding AH, Yates J, Sandhu MS, Higgins JP. The association between the peroxisome proliferator-activated receptor-gamma2 (PPARG2) Pro12Ala gene variant and type 2 diabetes mellitus: A HuGE review and meta-analysis. Am J Epidemiol. 2010;171(6):645-55. [View at Publisher] [DOI] [Google Scholar]
15. McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363(24):2339-50. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Langenberg C, Lotta LA. Genomic insights into the causes of type 2 diabetes. Lancet. 2018;391(10138):2463-74. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-43. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54(12):3541-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: Glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141(6):421-31. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation. 2002;106(16):2067-72. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Sarafidis PA, Stafylas PC, Georgianos PI, Avramides A, Lasaridis AN. Effect of thiazolidinediones on albuminuria and proteinuria in patients with type 2 diabetes: A meta-analysis. Am J Kidney Dis. 2010;55(5):835-47. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278(36):34268-76. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Ricote M, Huang JT, Welch JS, Schmedtje JF Jr, Liebman MN, Collins T, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391(6662):79-82. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J, et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000;26(1):76-80. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



