Volume 8, Issue 4 (Journal of Clinical and Basic Research (JCBR) 2024)
jcbr 2024, 8(4): 5-9 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Adebajo O, Ojo J, Bassey N, Adebajo P, Adeyemo A. Effects of Laurus nobilis on pregnancy and fetal growth using pregnant Sprague-Dawley. jcbr 2024; 8 (4) :5-9
URL: http://jcbr.goums.ac.ir/article-1-461-en.html
URL: http://jcbr.goums.ac.ir/article-1-461-en.html
1- College of Health Sciences, Bowen University, Iwo 232102, Osun State, Nigeria
2- College of Health Sciences, Bowen University, Iwo 232102, Osun State, Nigeria ; Department of Cell Biology and Genetics, Faculty of Science, University of Lagos, Akoka 100213, Lagos State, Nigeria ,basseynkereuweme@gmail.com
2- College of Health Sciences, Bowen University, Iwo 232102, Osun State, Nigeria ; Department of Cell Biology and Genetics, Faculty of Science, University of Lagos, Akoka 100213, Lagos State, Nigeria ,
Full-Text [PDF 785 kb]
(407 Downloads)
| Abstract (HTML) (1236 Views)
Discussion
Food and supplements consumed during pregnancy impact the health of expectant mothers and fetuses (28). Pregnancy outcomes depend on comprehensive prenatal care that promotes healthy nutritional habits (29). Pregnant women must understand the risks and advantages of various dietary choices (10). Considering the use of L. nobilis in a wide variety of dishes, there is a need to study the effects of bay leaves on female reproductive organs and fetal parameters.
Laurus nobilis treatment significantly affects fetal parameters, manifested as decreased litters, litter weight, and placental weight. Our study was in concordance with Sathasivam et al. (30) 's study, which reported a positive correlation between placental weight and birth weight. These may result from a significant decrease in the transfer of nutritional substances from the mother to the fetus. Although our study's sample size was small, our results indicate the nutritional value of L. nobilis.
Hormonal assays revealed a dose-dependent decrease in FSH and LH levels. However, there was a dose-dependent decrease in progesterone levels in the treatment groups compared with the control group. As reported by Bosch et al. (31), these hormonal deficits result from decreased gonadotropin production. This suggests that L. nobilis influences gonadotropin activity, which can impair implantation and embryo development in the early stages of pregnancy. This may also affect the mother's immune response, potentially leading to embryo rejection (32).
The treatment groups showed a decrease in SOD concentrations in the ovaries and uteri, similar to previous studies showing reduced activity with increased sucrose intake (33), while curcumin and capsaicin have been found to increase ovarian SOD levels (34). Lu et al. (35) proposed that suppressing SODs may hinder its protective effects against oxidative stress and oxygen species-mediated ailments.
Catalase expression levels showed a dose-dependent decrease in the ovary and uterus of the treatment groups compared to that in the control group. This contrasts with Kaygusuzoglu et al.'s study (36), which reported that increased catalase activity with zingerone treatment reduced oxidative DNA damage indicators and inhibited apoptosis. Repression of catalase concentrations may result in increased ovarian damage, exacerbated by apoptotic ovarian follicular cells, which decrease fecundity due to catalase suppression (37).
Administration of L. nobilis increased malondialdehyde levels in the ovaries and uteri, a finding consistent with that of Sadowska et al. (33). In contrast to Laurus nobilis, Chlorella vulgaris supplementation reduced malondialdehyde levels and could have protective effects against lipid-peroxidation-induced cell damage, as reported by Sikiru et al. (38). In our study, L. nobilis administration increased malondialdehyde levels, potentially damaging cell membranes.
The vasculature of the ovary plays a crucial role in tissue oxygenation, hormone transport, nutrient intake, and waste elimination (39). Low doses of L. nobilis do not cause adverse effects on ovarian tissue; however, increased dosages can cause vascular congestion in the ovarian stroma. Increased ovarian stromal vascularization has been linked to PCOS pathogenesis of polycystic ovary syndrome (40). High dosages of L. nobilis can cause endometrial gland proliferation and epithelial hyperplasia, which can affect uterine function and pregnancy, leading to pathological diseases such as endometrial hyperplasia and reduced female fertility (41). L. nobilis use during pregnancy causes uterine vascular congestion, which, according to Elagwany (42), is associated with pregnancy and uterine complications.
Conclusion
Extensive consumption of L. nobilis during pregnancy may negatively affect oxidative stress, hormonal levels, and the histological structure of the ovary and uterus. The mechanisms underlying these effects need to be elucidated, and the safety of L. nobilis consumption during pregnancy in humans must be determined. Hence, more clinical studies may be required to better comprehend the impact of L. nobilis on maternal and fetal health.
Acknowledgement
The authors thank the College of Health Sciences at Bowen University for providing the research facilities.
Funding sources
This study was done through the collaborative efforts of all the authors.
Ethical statement
This study was approved by the Bowen University Institutional Research Ethics Committee on June 1, 2023 (Reference number BUI/COHES/ANA/01013). The experimental animals were cared for according to the committee’s guidelines.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
OA: Contributed to research conceptualization and design, editing the initial draft of the article, and providing funding. JO: Performed the histological examination of ovaries and uteri and provided funding. NB: Collected data, wrote the initial and final draft of the article, and provided funding. PA: Performed data analysis and provided logistic support and funding. AA: Performed animal care and oral administration of L. nobilis, collected data, and provided funding. All authors critically reviewed and approved the final manuscript.
Full-Text: (298 Views)
Introduction
The female reproductive system is a complex network of internal and external organs (1). It produces gametes and certain sex hormones (2,3) and maintains the zygote throughout pregnancy until the mature fetus is ready for delivery (4). Pregnancy involves the gestation period from ovum fertilization to embryo implantation in the female body, ending with either spontaneous or voluntary abortions or delivery (5). Throughout pregnancy, the mother's body undergoes significant anatomical and physiological changes (6) to support the growing baby and prepare her for labor and delivery (7). Pregnancy also leads to hormonal, immunological, and metabolic changes in the woman's body, significantly affecting maternal and fetal health (8). Alterations in hormone levels affect the complex physiological adjustments required for fetal growth (9).
Nutrition, through diet choice, has immense short- and long-term effects on pregnancy health (10,11). An adequate nutritional regimen rich in macronutrients throughout pregnancy is essential for achieving the best possible birth outcomes, maternal health, and child development (11). Several maternal dietary factors have been explored with adverse pregnancy and child health and development outcomes (12).
Human nutrition relies heavily on nutrients derived from vegetables and herbs (13). Bay Leaf (Laurus nobilis), also known as bay laurel or Roman laurel (14), is an important herb used in meals, pharmaceuticals, and cosmetics (15). Grown in the southern Mediterranean and other parts of the world (16), bay leaf is a very aromatic and fragrant herb used as a condiment in soups, fish, meat products, stews, puddings, and beverages (17,18). L. nobilis' biological and pharmacologic properties include nematocidal, insecticidal, antibacterial, antifungal, and antioxidant properties (18). L. nobilis also boosts the immune system due to its antidiarrheal, anti-inflammatory, and anti-diabetic potentials (19). This medicinal herb has wound healing, anticonvulsant, antioxidant, antimicrobial, antiviral, and anticholinergic properties (18,20). It also has the potential to reduce the concentrations of uric acid and blood cholesterol (21).
Plants, herbs, and spices contain bioactive compounds that can affect oxidative stress (22). Oxidative stress has been implicated in female reproductive complications (23), such as follicular abnormalities, faulty meiosis, reduced fertilization rates, delayed embryonic development, and some diseases, such as polycystic ovarian syndrome and ovarian endometriosis cysts (24). It can also harm maternal and placental health, cause gestational diabetes mellitus, and potentially lead to fetal mortality (6). Oxidative stress accelerates ovarian and uterine aging (25,26).
L. nobilis and its essential oils are generally safe for consumption in small quantities for most people. In some parts of the world, pregnant women consume diets with high L. nobilis content because it is believed to strengthen pregnancy, promote fetal growth, and improve maternal and child health. However, there are no credible studies supporting the high consumption of L. nobilis by pregnant women (27). Hence, this study aimed to investigate the effects of L. nobilis on pregnancy and fetal growth using histological, hormonal, oxidative stress, and fetal parameters in a Sprague-Dawley rat model.
Methods
Experimental design and animal groups
Freshly harvested L. nobilis leaves were air-dried and ground into a powdery form for easy administration to rats in the treatment groups. Twenty-four (24) female pregnant Sprague-Dawley rats weighing (152 ± 29 g) were randomly assigned to four groups consisting of six (6) rats:
Group A: served as control group (Were administered with 10 ml distilled water)
Group B: low dose group (Administered with 50mg/kg L.nobilis)
Group C: medium dose group (Administered with 100mg/kg of ground L.nobilis)
Group D: High-dose group (200 mg/kg of ground L.nobilis).
The rats in the treatment groups were orally administered ground bay leaf mixed with water daily for 19 days (Within Sprague-Dawley rats' gestation period of 21-23 days) at the specified concentrations. All animals had ad libitum access to rat feed and clean water. The animals were housed in standard clean cages under moderately constant environmental conditions, proper aeration, and adequate lighting.
All procedures were carried out according to the standard international guidelines on animal use for research (National Research Council, 2011). The Animal Research Ethical Committee of Bowen University approved this study.
Animal sacrifice and sample collection
Pregnant Sprague-Dawley rats were fasted for 12 h before being sacrificed 20 d after oral administration. The ovaries, uteri, and fetuses were harvested for histological and biochemical analyses. Ovaries and uteri were dissected and fixed in Bouin's solution to prevent tissue degradation. Biochemical samples were homogenized and preserved on ice. Fetuses were measured for various parameters, including crown-rump length, tail length, fetal weight, umbilical cord length, and placental weight.
Biochemical analysis
Before the rats were sacrificed, 5 ml of blood was collected from their saphenous veins and stored at -20 °C for analysis. Serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and progesterone were determined in all pregnant rats in each group using enzyme-linked immunosorbent assay (ELISA) kits.
Histological procedure
The sections were dewaxed in two stages of xylene, with each stage lasting for 2 minutes. They were rehydrated in a series of descending concentrations of alcohol (100%, 95%, 80%, 70%, and 50%) for 2 minutes each. The tissues were rinsed in distilled water for 3 minutes and then stained with hematoxylin for 15-20 minutes. The sections were rinsed in running tap water for 1-5 minutes until they turned blue, then they were immersed in 70% ethanol containing 0.5% hydrochloric acid for about 5 seconds to remove the excess dye. The sections were rinsed in running tap water for 10-15 minutes and stained in eosin solution for 2-5 minutes. The excess eosin dye was rinsed off in running tap water for 1-5 minutes. Then, the sections were dehydrated in increasing alcohol concentrations, cleared in xylene, and mounted in a synthetic resin medium. Four micrometer-thick paraffin sections were created for microscopic examination.
Statistical analysis
The findings of this study were expressed using mean and standard deviation in the statistical analysis. Normality was determined using the SPSS software version 20. The mean difference between each group was evaluated using a one-way analysis of variance (ANOVA) compared to the control group at a significance level of 0.05.
Results
Body weight
As shown in Table 1, there was a high percentage increase in the weight of Group A rats (+38.0%) and a significant (p < 0.05) increase in Group B (+6.7%) and Group C (+9.4%) when compared to Group D (+2.7%).
The female reproductive system is a complex network of internal and external organs (1). It produces gametes and certain sex hormones (2,3) and maintains the zygote throughout pregnancy until the mature fetus is ready for delivery (4). Pregnancy involves the gestation period from ovum fertilization to embryo implantation in the female body, ending with either spontaneous or voluntary abortions or delivery (5). Throughout pregnancy, the mother's body undergoes significant anatomical and physiological changes (6) to support the growing baby and prepare her for labor and delivery (7). Pregnancy also leads to hormonal, immunological, and metabolic changes in the woman's body, significantly affecting maternal and fetal health (8). Alterations in hormone levels affect the complex physiological adjustments required for fetal growth (9).
Nutrition, through diet choice, has immense short- and long-term effects on pregnancy health (10,11). An adequate nutritional regimen rich in macronutrients throughout pregnancy is essential for achieving the best possible birth outcomes, maternal health, and child development (11). Several maternal dietary factors have been explored with adverse pregnancy and child health and development outcomes (12).
Human nutrition relies heavily on nutrients derived from vegetables and herbs (13). Bay Leaf (Laurus nobilis), also known as bay laurel or Roman laurel (14), is an important herb used in meals, pharmaceuticals, and cosmetics (15). Grown in the southern Mediterranean and other parts of the world (16), bay leaf is a very aromatic and fragrant herb used as a condiment in soups, fish, meat products, stews, puddings, and beverages (17,18). L. nobilis' biological and pharmacologic properties include nematocidal, insecticidal, antibacterial, antifungal, and antioxidant properties (18). L. nobilis also boosts the immune system due to its antidiarrheal, anti-inflammatory, and anti-diabetic potentials (19). This medicinal herb has wound healing, anticonvulsant, antioxidant, antimicrobial, antiviral, and anticholinergic properties (18,20). It also has the potential to reduce the concentrations of uric acid and blood cholesterol (21).
Plants, herbs, and spices contain bioactive compounds that can affect oxidative stress (22). Oxidative stress has been implicated in female reproductive complications (23), such as follicular abnormalities, faulty meiosis, reduced fertilization rates, delayed embryonic development, and some diseases, such as polycystic ovarian syndrome and ovarian endometriosis cysts (24). It can also harm maternal and placental health, cause gestational diabetes mellitus, and potentially lead to fetal mortality (6). Oxidative stress accelerates ovarian and uterine aging (25,26).
L. nobilis and its essential oils are generally safe for consumption in small quantities for most people. In some parts of the world, pregnant women consume diets with high L. nobilis content because it is believed to strengthen pregnancy, promote fetal growth, and improve maternal and child health. However, there are no credible studies supporting the high consumption of L. nobilis by pregnant women (27). Hence, this study aimed to investigate the effects of L. nobilis on pregnancy and fetal growth using histological, hormonal, oxidative stress, and fetal parameters in a Sprague-Dawley rat model.
Methods
Experimental design and animal groups
Freshly harvested L. nobilis leaves were air-dried and ground into a powdery form for easy administration to rats in the treatment groups. Twenty-four (24) female pregnant Sprague-Dawley rats weighing (152 ± 29 g) were randomly assigned to four groups consisting of six (6) rats:
Group A: served as control group (Were administered with 10 ml distilled water)
Group B: low dose group (Administered with 50mg/kg L.nobilis)
Group C: medium dose group (Administered with 100mg/kg of ground L.nobilis)
Group D: High-dose group (200 mg/kg of ground L.nobilis).
The rats in the treatment groups were orally administered ground bay leaf mixed with water daily for 19 days (Within Sprague-Dawley rats' gestation period of 21-23 days) at the specified concentrations. All animals had ad libitum access to rat feed and clean water. The animals were housed in standard clean cages under moderately constant environmental conditions, proper aeration, and adequate lighting.
All procedures were carried out according to the standard international guidelines on animal use for research (National Research Council, 2011). The Animal Research Ethical Committee of Bowen University approved this study.
Animal sacrifice and sample collection
Pregnant Sprague-Dawley rats were fasted for 12 h before being sacrificed 20 d after oral administration. The ovaries, uteri, and fetuses were harvested for histological and biochemical analyses. Ovaries and uteri were dissected and fixed in Bouin's solution to prevent tissue degradation. Biochemical samples were homogenized and preserved on ice. Fetuses were measured for various parameters, including crown-rump length, tail length, fetal weight, umbilical cord length, and placental weight.
Biochemical analysis
Before the rats were sacrificed, 5 ml of blood was collected from their saphenous veins and stored at -20 °C for analysis. Serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and progesterone were determined in all pregnant rats in each group using enzyme-linked immunosorbent assay (ELISA) kits.
Histological procedure
The sections were dewaxed in two stages of xylene, with each stage lasting for 2 minutes. They were rehydrated in a series of descending concentrations of alcohol (100%, 95%, 80%, 70%, and 50%) for 2 minutes each. The tissues were rinsed in distilled water for 3 minutes and then stained with hematoxylin for 15-20 minutes. The sections were rinsed in running tap water for 1-5 minutes until they turned blue, then they were immersed in 70% ethanol containing 0.5% hydrochloric acid for about 5 seconds to remove the excess dye. The sections were rinsed in running tap water for 10-15 minutes and stained in eosin solution for 2-5 minutes. The excess eosin dye was rinsed off in running tap water for 1-5 minutes. Then, the sections were dehydrated in increasing alcohol concentrations, cleared in xylene, and mounted in a synthetic resin medium. Four micrometer-thick paraffin sections were created for microscopic examination.
Statistical analysis
The findings of this study were expressed using mean and standard deviation in the statistical analysis. Normality was determined using the SPSS software version 20. The mean difference between each group was evaluated using a one-way analysis of variance (ANOVA) compared to the control group at a significance level of 0.05.
Results
Body weight
As shown in Table 1, there was a high percentage increase in the weight of Group A rats (+38.0%) and a significant (p < 0.05) increase in Group B (+6.7%) and Group C (+9.4%) when compared to Group D (+2.7%).
Number of litters and weight of the uterus, ovary, and litter
The weights of the ovaries did not differ significantly (p < 0.05) across the different groups. However, the weight of the uterus was markedly lower in Group B (Low-dose group) than in the other groups (Table 2). Additionally, litter weight was significantly decreased in the treatment groups (Groups B, C, and D) compared to the control group.
The weights of the ovaries did not differ significantly (p < 0.05) across the different groups. However, the weight of the uterus was markedly lower in Group B (Low-dose group) than in the other groups (Table 2). Additionally, litter weight was significantly decreased in the treatment groups (Groups B, C, and D) compared to the control group.
|
Table 2. Number of litters and weights of uterus, ovary, and litter of pregnant Sprague Dawley rats after the oral administration of L. nobilis
.PNG) |
Fetal parameters
As shown in Table 3, there was a significant (p < 0.05) decrease in the fetal parameters between the control group (Group A) and the treatment groups (Groups B, C, and D). The reduction of crown-rump length and umbilical cord length was dose-dependent. The tail length and placenta weight showed a dose-dependent increase, with the high-dose group (Group D) having the highest measurements significantly lower than the control group (Group A).
As shown in Table 3, there was a significant (p < 0.05) decrease in the fetal parameters between the control group (Group A) and the treatment groups (Groups B, C, and D). The reduction of crown-rump length and umbilical cord length was dose-dependent. The tail length and placenta weight showed a dose-dependent increase, with the high-dose group (Group D) having the highest measurements significantly lower than the control group (Group A).
|
Table 3. Measurements of fetal parameters of Sprague Dawley rats after oral administration of L. nobilis
.PNG) |
Hormonal assay
The analysis of FSH, LH, and progesterone revealed a significant (p<0.05) decrease in the serum levels of these hormones in the treatment groups compared with the control group. It was observed that the hormonal (FSH, LH, and progesterone) levels of the treatment groups decreased with increased dosage of the grounded bay leaves administered (Table 4).
The analysis of FSH, LH, and progesterone revealed a significant (p<0.05) decrease in the serum levels of these hormones in the treatment groups compared with the control group. It was observed that the hormonal (FSH, LH, and progesterone) levels of the treatment groups decreased with increased dosage of the grounded bay leaves administered (Table 4).
|
Table 4. Mean and standard error of FSH, LH, and progesterone serum levels in the different groups after administration of L. nobilis.
.PNG) |
Biochemical assay
Ovary: Biochemical analysis (Table 5) revealed significantly lower levels of superoxide dismutase and catalase in the treatment groups than in the control group, indicating higher levels of oxidative stress markers in the ovary (p < 0.05). Conversely, malondialdehyde expression levels were significantly (p < 0.05) higher in the treatment groups than in the control group. The decrease in relative expression levels of superoxide dismutase and catalase in the treatment groups was dose-dependent. Similarly, the increase in the expression levels of malondialdehyde in the treatment groups was dose-dependent.
Uterus: Biochemical analysis of the uteri for oxidative stress markers revealed a similar pattern to that observed in the ovary, as indicated in Table 5. The relative expression levels of these markers in the uteri indicated that superoxide dismutase and catalase were significantly lower in the treatment groups than in the control group. In contrast, malondialdehyde levels were significantly higher in the treatment groups than in the control group. Significant (p < 0.05) differences in the expression levels of these markers were all dose-dependent.
Ovary: Biochemical analysis (Table 5) revealed significantly lower levels of superoxide dismutase and catalase in the treatment groups than in the control group, indicating higher levels of oxidative stress markers in the ovary (p < 0.05). Conversely, malondialdehyde expression levels were significantly (p < 0.05) higher in the treatment groups than in the control group. The decrease in relative expression levels of superoxide dismutase and catalase in the treatment groups was dose-dependent. Similarly, the increase in the expression levels of malondialdehyde in the treatment groups was dose-dependent.
Uterus: Biochemical analysis of the uteri for oxidative stress markers revealed a similar pattern to that observed in the ovary, as indicated in Table 5. The relative expression levels of these markers in the uteri indicated that superoxide dismutase and catalase were significantly lower in the treatment groups than in the control group. In contrast, malondialdehyde levels were significantly higher in the treatment groups than in the control group. Significant (p < 0.05) differences in the expression levels of these markers were all dose-dependent.
|
Table 5. Biochemical assay of malondialdehyde, superoxide dismutase, and catalase in ovarian and uterine serum after administration of L. nobilis.
.PNG) |
Histological Examination
Photomicrographs of sections of the ovary
The ovary photomicrographs of the control group (Figure 1) and different concentrations of L. nobilis (Figures 2, 3, and 4) are shown in Figures 1-4.
Photomicrographs of sections of the ovary
The ovary photomicrographs of the control group (Figure 1) and different concentrations of L. nobilis (Figures 2, 3, and 4) are shown in Figures 1-4.
|
Control group
.PNG) Figure 1. Normal antral follicles (White arrow) with normal theca cells (Blue arrow) within the ovarian cortex and degenerated corpus luteum (Slender arrow) in the control group. Low-dose treatment group .PNG) Figure 2. Normal antral and Graafian follicles (White arrow) with normal luteinized stromal cells and normal connective tissue (Slender arrow) in the ovaries of the low-dose treatment group. Medium-dose treatment group .PNG) Figure 3. Normal antral follicles (White arrow) with normal theca cells (Blue arrow) within the ovarian cortex, luteinized stromal cells, and mild vascularization (Slender arrow) in the ovary of the medium-dose treatment group. High-dose treatment group .PNG) Figure 4. Normal antral follicles (White arrow) with normal theca cells (Blue arrow) within the ovarian cortex and mild vascular congestion of the ovarian stroma (Slender arrow) in the ovarian section of the high-dose treatment group. |
Photomicrographs of sections of the uterus
The uterus photomicrographs of the control group (Figure 5) and different concentrations of L. nobilis (Figures 6, 7, and 8) are shown in Figures 5-8.
The uterus photomicrographs of the control group (Figure 5) and different concentrations of L. nobilis (Figures 6, 7, and 8) are shown in Figures 5-8.
|
Control group
.PNG) Figure 5. Normal endometrial epithelial layer (White arrow), normal endometrial gland (Blue arrow), and moderate infiltration of endometrial stroma by inflammatory cells (Slender arrow) in the uterine section of the control group. Low-dose treatment group .PNG) Figure 6. Normal endometrial epithelial layer (White arrow), normal endometrial gland (Blue arrow) with severe infiltration of the endometrial stroma by inflammatory cells (Slender arrow), and mild vascular congestion of the myometrium (Red arrow) in the uterine section of the low-dose group. Medium-dose treatment group 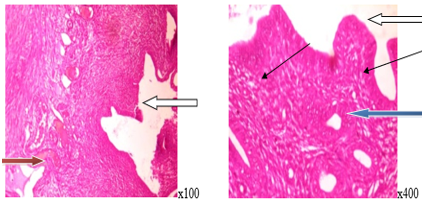 Figure 7. Normal endometrial epithelial layer (White arrow), normal endometrial gland (Blue arrow) with moderate infiltration of inflammatory cells (Slender arrow), and mild vascular congestion (Red arrow) in the uterine section of the medium-dose group. High-dose treatment group .PNG) Figure 8. Normal endometrial epithelial layer (White arrow), moderate proliferation of endometrial gland (Blue arrow) with epithelial hyperplasia, and severe infiltration of the endometrial stroma by inflammatory cells (Slender arrow) in the uterine section of the high-dose group. |
Discussion
Food and supplements consumed during pregnancy impact the health of expectant mothers and fetuses (28). Pregnancy outcomes depend on comprehensive prenatal care that promotes healthy nutritional habits (29). Pregnant women must understand the risks and advantages of various dietary choices (10). Considering the use of L. nobilis in a wide variety of dishes, there is a need to study the effects of bay leaves on female reproductive organs and fetal parameters.
Laurus nobilis treatment significantly affects fetal parameters, manifested as decreased litters, litter weight, and placental weight. Our study was in concordance with Sathasivam et al. (30) 's study, which reported a positive correlation between placental weight and birth weight. These may result from a significant decrease in the transfer of nutritional substances from the mother to the fetus. Although our study's sample size was small, our results indicate the nutritional value of L. nobilis.
Hormonal assays revealed a dose-dependent decrease in FSH and LH levels. However, there was a dose-dependent decrease in progesterone levels in the treatment groups compared with the control group. As reported by Bosch et al. (31), these hormonal deficits result from decreased gonadotropin production. This suggests that L. nobilis influences gonadotropin activity, which can impair implantation and embryo development in the early stages of pregnancy. This may also affect the mother's immune response, potentially leading to embryo rejection (32).
The treatment groups showed a decrease in SOD concentrations in the ovaries and uteri, similar to previous studies showing reduced activity with increased sucrose intake (33), while curcumin and capsaicin have been found to increase ovarian SOD levels (34). Lu et al. (35) proposed that suppressing SODs may hinder its protective effects against oxidative stress and oxygen species-mediated ailments.
Catalase expression levels showed a dose-dependent decrease in the ovary and uterus of the treatment groups compared to that in the control group. This contrasts with Kaygusuzoglu et al.'s study (36), which reported that increased catalase activity with zingerone treatment reduced oxidative DNA damage indicators and inhibited apoptosis. Repression of catalase concentrations may result in increased ovarian damage, exacerbated by apoptotic ovarian follicular cells, which decrease fecundity due to catalase suppression (37).
Administration of L. nobilis increased malondialdehyde levels in the ovaries and uteri, a finding consistent with that of Sadowska et al. (33). In contrast to Laurus nobilis, Chlorella vulgaris supplementation reduced malondialdehyde levels and could have protective effects against lipid-peroxidation-induced cell damage, as reported by Sikiru et al. (38). In our study, L. nobilis administration increased malondialdehyde levels, potentially damaging cell membranes.
The vasculature of the ovary plays a crucial role in tissue oxygenation, hormone transport, nutrient intake, and waste elimination (39). Low doses of L. nobilis do not cause adverse effects on ovarian tissue; however, increased dosages can cause vascular congestion in the ovarian stroma. Increased ovarian stromal vascularization has been linked to PCOS pathogenesis of polycystic ovary syndrome (40). High dosages of L. nobilis can cause endometrial gland proliferation and epithelial hyperplasia, which can affect uterine function and pregnancy, leading to pathological diseases such as endometrial hyperplasia and reduced female fertility (41). L. nobilis use during pregnancy causes uterine vascular congestion, which, according to Elagwany (42), is associated with pregnancy and uterine complications.
Conclusion
Extensive consumption of L. nobilis during pregnancy may negatively affect oxidative stress, hormonal levels, and the histological structure of the ovary and uterus. The mechanisms underlying these effects need to be elucidated, and the safety of L. nobilis consumption during pregnancy in humans must be determined. Hence, more clinical studies may be required to better comprehend the impact of L. nobilis on maternal and fetal health.
Acknowledgement
The authors thank the College of Health Sciences at Bowen University for providing the research facilities.
Funding sources
This study was done through the collaborative efforts of all the authors.
Ethical statement
This study was approved by the Bowen University Institutional Research Ethics Committee on June 1, 2023 (Reference number BUI/COHES/ANA/01013). The experimental animals were cared for according to the committee’s guidelines.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
OA: Contributed to research conceptualization and design, editing the initial draft of the article, and providing funding. JO: Performed the histological examination of ovaries and uteri and provided funding. NB: Collected data, wrote the initial and final draft of the article, and provided funding. PA: Performed data analysis and provided logistic support and funding. AA: Performed animal care and oral administration of L. nobilis, collected data, and provided funding. All authors critically reviewed and approved the final manuscript.
Article Type: Research |
Subject:
Basic medical sciences
References
1. Rendi MH, Muelenbachs A, Garcia RL, Boyd KL. Female Reproductive System. In: Treuting PM, Dintzis SM, Montine KS, editors. Comparative Anatomy and Histology. London, England: Academic Press; 2017. p.253-84. [View at Publisher] [DOI] [Google Scholar]
2. Ramírez-González JA, Vaamonde-Lemos R, Cunha-Filho JS, Varghese AC, Swanson RJ. Overview of the Female Reproductive System. In: Vaamonde D, du Plessis SS, Agarwal A, editors. Exercise and Human Reproduction. New York: Springer; 2016. pp.19-46. [View at Publisher] [DOI] [Google Scholar]
3. Vue Z, Mullen RD, Yen S, Ontiveros AE, Stewart AC, Behringer RR. Fetal and Postnatal Female Tract Development. In: Skinner MK, editor. Encyclopedia of Reproduction. Amsterdam: Academic Press; 2018. pp.261-8. [View at Publisher] [DOI] [Google Scholar]
4. Guyton AC, Hall JE. Textbook of Medical Physiology. 14th ed. Philadelphia: Elsevier; 2021. pp.1091. [View at Publisher] [Google Scholar]
5. El-Mazny A. Female Reproductive System: Clinical Anatomy and Physiology. South Carolina: CreateSpace Independent Publishing; 2016. pp.119. [View at Publisher]
6. Toboła-Wróbel K, Pietryga M, Dydowicz P, Napierała M, Brązert J, Florek E. Association of Oxidative Stress on Pregnancy. Oxid Med Cell Longev. 2020;2020:6398520. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Bhatia P, Chhabra S. Physiological and anatomical changes of pregnancy: Implications for anaesthesia. Indian J Anaesth. 2018;62(9):651-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Rezaee R, Ravangard R, Amani F, Tafti AD, Shokrpour N, Bahrami MA. Healthy lifestyle during pregnancy: Uncovering the role of online health information seeking experience. PLOS ONE. 2022;17(8):e0271989. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Motosko CC, Bieber AK, Pomeranz MK, Stein JA, Martires KJ. Physiologic changes of pregnancy: A review of the literature. Int J Womens Dermatol. 2017;3(4):219-24. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Grenier LN, Atkinson SA, Mottola MF, Wahoush O, Thabane, L, Xie, F, et al. Be Healthy in Pregnancy: Exploring factors that impact pregnant women's nutrition and exercise behaviours. Matern Child Nutr. 2021;17(1):e13068. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Marshall N, Abrams B, Barbour L, Catalano P, Christian P, Friedman J, et al. The importance of nutrition in pregnancy and lactation: Lifelong consequences. Am J Obstet Gynecol. 2022;226(5):607-32. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Naaz A, Muneshwar KN. How Maternal Nutritional and Mental Health Affects Child Health During Pregnancy: A Narrative Review. Cureus. 2023;15(11):e48763. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Mishra S, Lakhawat S. Roles of herbs in human nutrition. Just Agric. 2021;1(11):1-4. [View at Publisher] [Google Scholar]
14. Dobroslavić E, Repajić M, Dragović-Uzelac V, Elez Garofulić I. Isolation of Laurus nobilis Leaf Polyphenols: A Review on Current Techniques and Future Perspectives. Foods. 2022;11(2):235. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Kara H, Bayir A, Korkmaz H, Talay F, Ak A. Hepatotoxicity caused by bay leaf (Laurus nobilis): A case report. J Emerg Med Case Rep. 2021;12(4):124-6. [View at Publisher] [DOI] [Google Scholar]
16. Obiandu C, Laz-Okenwa JOA, Owhorji BI, Tamuno-Opubo A, Asuzu-Samuel HO. Evaluation of the effects of extracts of Laurus nobilis on some biochemical parameters of Wistar rats. Sch Int J Anat Physiol. 2023;6(4):37-41. [View at Publisher] [DOI] [Google Scholar]
17. Mansour O, Darwish M, Ismail G, Douba Z, Ismaeel A, Eldair KS. Review Study on the Physiological Properties and Chemical Composition of the Laurus nobilis. Pharm Chem J. 2018;5(1):225-31. [View at Publisher] [Google Scholar]
18. Paparella A, Nawade B, Shaltiel-Harpaz L, Ibdah M. A review of the botany, volatile composition, biochemical and molecular aspects, and traditional uses of Laurus nobilis. Plants (Basel). 2022;11(9):1209. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Bommakanti V, Ajikumar AP, Sivi CM, Prakash G, Mundanat AS, Ahmad F, et al. An Overview of Herbal Nutraceuticals, Their Extraction, Formulation, Therapeutic Effects and Potential Toxicity. Separations. 2023;10(3):177. [View at Publisher] [DOI] [Google Scholar]
20. Brinza I, Boiangiu RS, Hancianu M, Cioanca O, Orhan IE, Hritcu L. Bay Leaf (Laurus nobilis L.) Incense Improved Scopolamine-Induced Amnesic Rats by Restoring Cholinergic Dysfunction and Brain Antioxidant Status. Antioxidants (Basel). 2021;10(2):259. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Ju B, Chen B, Zhang X, Han C, Jiang A. Purification and Characterization of Bioactive Compounds from Styela clava. J Chem. 2014;2014(1):525141. [View at Publisher] [DOI] [Google Scholar]
22. Anand S, Bharadvaja N. Potential Benefits of Nutraceuticals for Oxidative Stress Management. Rev Bras Farmacogn. 2022;32(2):211-20. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Immediata V, Ronchetti C, Spadaro D, Cirillo F, Levi-Setti PE. Oxidative Stress and Human Ovarian Response-From Somatic Ovarian Cells to Oocytes Damage: A Clinical Comprehensive Narrative Review. Antioxidants (Basel). 2022;11(7):1335. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Wang L, Tang J, Wang L, Tan F, Song H, Zhou J, et al. Oxidative stress in oocyte aging and female reproduction. J Cell Physiol. 2021;236(12):7966-83. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Yang Z, Wei ML, Dong XY. Effects of Yu Linzhu on ovarian function and oocyte mitochondria in natural aging mice. Aging (Albany NY). 2021;13(19):23328-37. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Yan F, Zhao Q, Li Y, Zheng Z, Kong X, Shu C, et al. The role of oxidative stress in ovarian aging: a review. J Ovarian Res. 2022;15(1):100. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Damayanti D, Latifah L, Jati S, Runjati. The potential minerals of Bay leaf (Syzygium Polyanthum): To Support Woman in Pregnancy and Breastfeeding. Adv Health Sci Res (Atlantis Press). 2021;34:66-70. [View at Publisher] [DOI] [Google Scholar]
28. Jones HE, Fielder A. Neonatal abstinence syndrome: Historical perspective, current focus, future directions. Prev Med. 2015;80:12-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Santiago SE, Park GH, Huffman KJ. Consumption habits of pregnant women and implications for developmental biology: a survey of predominantly Hispanic women in California. Nutr J. 2013;12:91. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Sathasivam R, Selliah P, Sivalingarajah R, Mayorathan U, Munasinghe BM. Placental weight and its relationship with the birth weight of term infants and body mass index of the mothers. J Int Med Res. 2023;51(5):03000605231172895. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Bosch E, Alviggi C, Lispi M, Conforti A, Hanyaloglu AC, Chuderland D, et al. Reduced FSH and LH action: implications for medically assisted reproduction. Hum Reprod. 2021;36(6):1469-80. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Dante G, Vaccaro V, Facchinetti F. Use of progestagens during early pregnancy. Facts Views Vis Obgyn. 2013;5(1):66-71. [View at Publisher] [PMID] [Google Scholar]
33. Sadowska J, Dudzińska W, Dziaduch I. Effects of different models of sucrose intake on the oxidative status of the uterus and ovary of rats. PLOS ONE. 2021;16(5):e0251789. [View at Publisher] [DOI] [PMID] [Google Scholar]
34. Melekoglu R, Ciftci O, Eraslan S, Cetin A, Basak N. Beneficial effects of curcumin and capsaicin on cyclophosphamide-induced premature ovarian failure in a rat model. J Ovarian Res. 2018;11(1):33. [View at Publisher] [DOI] [PMID] [Google Scholar]
35. Lu J, Wang Z, Cao J, Chen Y, Dong Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2018;16(1):80. [View at Publisher] [DOI] [PMID] [Google Scholar]
36. Kaygusuzoglu E, Caglayan C, Kandemir FM, Yıldırım S, Kucukler S, Kılınc MA, et al. Zingerone ameliorates cisplatin‐induced ovarian and uterine toxicity via suppression of sex hormone imbalances, oxidative stress, inflammation and apoptosis in female wistar rats. Biomed Pharmacother. 2018;102:517-30. [View at Publisher] [DOI] [PMID] [Google Scholar]
37. Scalercio SR, Amorim CA, Brito DC, Percário S, Oskam IC, Domingues SFS, et al. Trolox enhances follicular survival after ovarian tissue autograft in squirrel monkey (Saimiri collinsi). Reprod Fertil Dev. 2015;28(11):1854-64. [View at Publisher] [DOI] [PMID] [Google Scholar]
38. Sikiru AB, Arangasamy A, Alemede IC, Guvvala PR, Egena SSA, Ippala JR, et al. Chlorella vulgaris supplementation effects on performances, oxidative stress and antioxidant genes expression in liver and ovaries of New Zealand White rabbits. Heliyon. 2019;5(9):e02470. [View at Publisher] [DOI] [PMID] [Google Scholar]
39. Kinnear HM, Tomaszewski CE, Chang FL, Moravek MB, Xu M, Padmanabhan V, et al. The ovarian stroma as a new frontier. Reproduction. 2020;160(3):R25-39. [View at Publisher] [DOI] [PMID] [Google Scholar]
40. Di Pietro M, Pascuali N, Parborell F, Abramovich D. Ovarian Angiogenesis in Polycystic Ovary Syndrome. Reprod. 2018;155(5):REP-17-0597. [View at Publisher] [DOI] [PMID] [Google Scholar]
41. Gao Y, Li S, Li Q. Uterine epithelial cell proliferation and endometrial hyperplasia: evidence from a mouse model. Mol Hum Reprod. 2014;20(8):776-86. [View at Publisher] [DOI] [PMID] [Google Scholar]
42. Elagwany AS. Pelvic congestion, enhanced myometrial vascularity, AVM versus normal vasculature variants: a confusing diagnosis regarding uterocervical vasculature. Indian J Surg Oncol. 2020;11(Suppl2):323-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).





.PNG)
