Volume 8, Issue 2 (Journal of Clinical and Basic Research (JCBR) 2024)
jcbr 2024, 8(2): 10-16 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mobisson S K, Godson C B, Iyanyi U L, Ehigiator B E, Obembe A O. Hematotoxic ameliorative potential of hydromethanol extract of Justica secunda and astymin in 2, 4-dinitrophenylhydrazine-induced anemic male wistar rats. jcbr 2024; 8 (2) :10-16
URL: http://jcbr.goums.ac.ir/article-1-445-en.html
URL: http://jcbr.goums.ac.ir/article-1-445-en.html
Samuel Kelechi Mobisson1 

 , Chiamaka Blossom Godson1
, Chiamaka Blossom Godson1 

 , Uchechukwu Loveth Iyanyi *2
, Uchechukwu Loveth Iyanyi *2 

 , Ben Enoluomen Ehigiator3
, Ben Enoluomen Ehigiator3 

 , Agona Odeh Obembe4
, Agona Odeh Obembe4 




 , Chiamaka Blossom Godson1
, Chiamaka Blossom Godson1 

 , Uchechukwu Loveth Iyanyi *2
, Uchechukwu Loveth Iyanyi *2 

 , Ben Enoluomen Ehigiator3
, Ben Enoluomen Ehigiator3 

 , Agona Odeh Obembe4
, Agona Odeh Obembe4 


1- Department of Human Physiology, Faculty of Basic Medical Sciences, Madonna University, Elele, Rivers State, Nigeria
2- Department of Pharmacology and Toxicology, Faculty of Pharmacy, Madonna University, Elele, Rivers State, Nigeria ,uchechukwuiyanyi@yahoo.com
3- Department of Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, Edo State University, Uzairue, Nigeria
4- Department of Human Physiology, Faculty of Basic Medical Sciences, University of Calabar, Cross River State, Nigeria
2- Department of Pharmacology and Toxicology, Faculty of Pharmacy, Madonna University, Elele, Rivers State, Nigeria ,
3- Department of Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, Edo State University, Uzairue, Nigeria
4- Department of Human Physiology, Faculty of Basic Medical Sciences, University of Calabar, Cross River State, Nigeria
Full-Text [PDF 675 kb]
(964 Downloads)
| Abstract (HTML) (3226 Views)
Full-Text: (1139 Views)
Introduction
According to the World Health Organization, globally, 40% of pregnant women and 42% of children under the age of five are anemic. Pregnant women and young children are disproportionately affected by anemia, a major global health issue (1). There are various forms of anemia, many of which are uncommon, but they are all characterized by a decrease in the quantity of hemoglobin and red blood cells in the blood (2). Hemolytic anemia develops when red blood cells are lost more quickly than the bone marrow can produce them. Cancer, autoimmune diseases, and other illnesses may be the cause, which determines the therapy (2). The body may produce acquired hemolytic anemia if it receives a signal to destroy healthy red blood cells (RBCs). Issues with the genes that regulate red blood cells are linked to inherited hemolytic anemia. Numerous internal and external factors can contribute to hemolytic anemia, including medicines related to the destruction of red blood cells. Hemolytic anemia is a feature of the clinical condition that is often associated with intoxication (3). Iron deficiency anemia is the most common single-nutrient deficiency disease (4). Iron deficiency may occur in increased demand, decreased intake, decreased or malabsorption, or chronic blood loss (5).
In 1895, Hermann Emil Fischer discovered the chemical compound phenylhydrazine, also known as hydroxybenzene. It is mainly used as a chemical intermediate in the pharmaceutical, agrochemical, and chemical industries (6). Once employed as antipyretics, phenylhydrazine and its derivatives have been shown to have toxic effects on blood cells; as a result, there is limited use of this compound as a medication. Instead, it is currently used as a chemical to induce anemia in animal studies (7). This results in a decrease in RBCs, HB, and hematocrit as well as an increase in the population of reticulocytes and the concentration of heme in serum.
In addition to being used as food, medicinal plants are the leading supplier of trade medicines. Despite advancements in the use of Western medications in healthcare delivery systems, medicinal plants remain an essential component of healthcare systems for both humans and animals. Approximately 80% of the world's population still depends on medicinal plants for basic medical requirements (8). Since medicinal plants have been found to possess bioactive molecules termed phytochemicals (9) and secondary metabolites that can protect humans against diseases (10), they have been reported as having therapeutic qualities utilized for the management of many disorders (10). It has been found that Justicia secunda works well for treating anemia (11). Justicia secunda is a member of the Acanthaceae family, also referred to as "sanguinaria" in Venezuela and "bloodroot" in Barbados. It is referred to as "obarabundu" locally in southeast Nigeria. It is known as "asindiri" or "ohowaazara" by the Ogbia people of Otuoke-Otuaba, Bayelsa, Niger-Delta region of Nigeria (12). J. secunda leaves are utilized in traditional medicine for wounds, anemia, and abdominal pain. It has been shown that the leaves of Justicia secunda exhibit hematinic, antisickling, antibacterial, and antihypertensive properties (13). The presence of tannins, flavonoids, alkaloids, quinines, anthocyanins, luteolin, anandamide, acetate, auranamide, quindoline, and pyrrolidone derivatives has been revealed by phytochemical screening of the plant (14).
A dietary supplement called astymin is crucial for children's growth, brain development, alertness, and overall health. Adults who use astymin report feeling less stressed about everyday life, having a stronger immune system, and recovering from illness more quickly (15). Astymin provides the body with essential amino acids needed to make protein, which helps build the body’s immune system. It also speeds up physical and mental growth in children, combats stress, quickens recovery from illness and allows the body to fight infection (15). This novel study aims to ascertain scientifically the hematopoietic potentials of Justicia secunda leaf in folklore medicine using phenylhydrazine-induced anemic Wistar rats.
Methods
Drugs and chemicals
The drugs and chemicals used included methanol, formalin, chloroform (Guangzhou JHD Chemical Reagent Co., Ltd. Shantou Guangdong, China), 2, 4-dinitrophenyl-hydrazine (Sigma-Aldrich Limited Germany), and astymin (Bayer Company, Inc., Leverkusen, Germany).
Hydro-methanol preparation of Justicia secunda
Fresh leaves of Justicia secunda were obtained and sent to a Botanist in the Faculty of Pharmacy, Madonna University for identification. They were dried at room temperature and ground to powder. For 48 hours, 590g of ground-up Justicia secunda was steeped in 1450 mL of methanol and 750 mL of water using an electronic weighing balance (Doran Scales, Inc., Batavia, Illinois, USA). The Whatman filter paper was used to sieve and filter the resulting suspension. Subsequently, the filtrate was concentrated in an electronic laboratory incubator (Labotech International Co., Ltd, Tokyo, Japan) at 60 °C. The jelly-like residue was introduced into an air-tight container and stored in the refrigerator until used. The LD50 of J. secunda was estimated to be 3800mg/kg body weight, as reported by previous studies (8). Hydromethanolic extraction was chosen to extract both hydrophilic and hydrophobic compounds of J. secunda.
Laboratory animals
For this study, we used twenty male Wistar rats weighing between 90 and 170g. The animals were housed in the Department of Physiology Animal House, University of Calabar, Nigeria. The animals were housed in standard animal cages (435 x 290 x 150 mm) with bedding made of wood shavings (5 rats per cage). They were subjected to a 12-hour light/dark cycle and given unlimited access to fresh water and feed (Provided by AEC Agrosystem Limited, Port Harcourt, and Rivers State, Nigeria). The National Committee for Research Ethics in Science and Technology (NENT), 2018, approved the guidelines for animal care, and they were acclimated to the conditions for seven days. The research protocol was approved by the University of Calabar's Animal Ethics Committee under approval number 040PHY3719.
Experimental design
The animals were randomly divided into 5 distinct groups (n = 4). At the end of seven days of acclimatization, 2, 4-dinitrophenyl-hydrazine, astymin, and J. secunda extract administration began. The treatment groups (B to E) received the drugs orally via gavage (Dosage per rat listed in Table 1) once a day, using the doses listed in Table 1. In contrast, the control group received feed and 0.5 ml of normal saline as a vehicle. Astymin and J. secunda extract administration lasted for 14 days, whereas 2, 4-dinitrophenyl-hydrazine was given intraperitoneal for just two days. Subsequently, the rats were anesthetized with chloroform, and blood samples were collected via ocular puncture. The samples were stored in an ice pack and immediately utilized to analyze hematologic parameters and serum oxidative stress markers.
Evaluation of hematologic indices
Packed cell volume (PCV), total white blood cell count (TWBC), platelet count, and red cell count were the tested hematological parameters.
PCV: The ratio (L/L) representing the percentage of red blood cells in total blood is the packed cell volume. To measure PCV, ¾ of the capillary tube was filled with well-mixed EDTA blood. The empty end was sealed, the tube was placed in a microhematocrit rotor, and centrifugation was performed for five minutes. Using the microhematocrit reader, the PCV was read immediately following centrifugation. To calculate hemoglobin, the PCV number was divided by three HB units or mg/dl. The concentration of Hb in g/l in one liter of packed red blood cells was provided by the mean cell hemoglobin concentration (MCHC). MCHEg/L equals Hb. Red cell size is indicated by mean cell volume (MCV), which is expressed in femtolitres (Fl). The amount of hemoglobin in an average red cell picogram (pg) was calculated using mean cell hemoglobin (MCH). This technique had been applied previously (16).
Assay for RBC Count X1012/L
A procedure described in the literature (16) was used. Formal citrate (Diluting fluid) containing approximately 4.0 ml was measured and poured into a test tube. After mixing thoroughly, 0.02 milliliters of EDTA blood were added. After the counting chamber was put together and loaded with thoroughly blended samples, it was examined undisturbed using an x10 objective lens. The quantity of red cells per liter was determined by counting the cells in little squares.
RBC count = N x 201 X109. Where N=Number counted, 201 is the diluting factor, 0.2mm2=Area. 0.1mm = depth of the chamber.
Assay for total WBC count unit*109/L
The procedure described in the literature was followed (16). A test tube was filled with 0.38 ml of dilution fluid, then 0.02 ml of thoroughly mixed EDTA blood was added and stirred. The counting chamber was put together, and a Pasteur pipette was used to re-mix the diluted blood sample. The sample was placed into one of the chamber's grids. The white cells were allowed to settle in the compartment by remaining undisturbed for 20 mm. An X10 objective lens was used to inspect the sample. Four huge squares of the chamber were counted to determine the number of white cells per liter.
WBC count (Per liter) = N x Df X 106/A*D
N: number of cells counted, Df: Dilution factor, A: area counted, D: chamber depth.
Assay for platelet count unit *109/L
The procedure outlined in the literature (16) was followed. A test tube was filled with 0.38 ml of dilution fluid, then 0.02 ml of thoroughly mixed EDTA blood was added and stirred. The well-mixed sample was placed into the counting chamber after it had been built. The chamber was left undisturbed for 20mms. To prevent the fluid from drying, the chamber was placed in a Petri dish on dampened tissue and was covered with a lid.
An X10 objective lens was used for inspection, the platelets were counted in little squares, and the number of platelets per liter was noted.
Determination of serum urea
Reagent R1 (0.1 ml) was pipetted into each tube. An amount of 10 u of the sample, standard, and D/W was added into the tubes, mixed, and incubated at 37'c for 10 minutes. A volume of 2.5 mL of R2 and R3 was pipetted into each tube, mixed, and incubated at 25° C for 15 minutes. Subsequently, they were read and recorded the absorbance at 546 nm. This was done using the method described in the literature (17). Test tubes were labeled as test, standard, and blank.
Determination of serum creatinine
The procedure outlined in the literature was followed (17). Three labels were placed on the test, standard, and blank test tubes, and the reagent (2 mL) was pipetted into each tube. After adding 0.1 ml of each sample, standard, and D/W to the tubes and mixing, the absorbance of the sample and standard was measured after 30 seconds. The absorbance of the sample and standard was measured two minutes later-A2 for the sample and standard. A1-A2 equaled D.
Determination of serum total protein (Biuret method) unit g/l
The procedure outlined by the literature (18) was followed. The labels of the test tubes were blank, sample, and standard. One milliliter of the reagent was pipetted into each tube. The appropriate tube was filled with 0.02 ml of the standard, sample, and D/W, mixed, and incubated for 30 minutes at 25 °C. The blank, zero were utilized at 540 nm, then they were read and the absorption was recorded.
Determination of bilirubin (Jendrasik and Grof method) unit: Umol/L
The procedure outlined by previous studies was followed (18). Labels of the test tubes were blank, sample, and standard. Each tube was pipetted with 0.2 ml of the reagent, and then a drop of sodium nitrate was added to the tube labeled as the sample. The tubes were filled with 0.02 mL of sample and 1 mL of caffeine, stirred, and then incubated for 10 minutes at 25 °C. After mixing each tube with 1 ml of tartrate, the tubes were incubated at 25 °C for 5 to 30 minutes. The absorbance at 590 nm was set to zero.
Determination of serum albumin (Bromocresol Green method) unit g/L
The procedure outlined by previous studies (18) was followed. The labels of the test tubes were blank, sample, and standard. Three milliliters of the albumin reagent were pipetted into each tube. Intro-appropriate containers were filled with 0.01 mL of the sample, mixed, and incubated for 10 minutes at 25°C. The blank was used to zero the spectrophotometer at 578nm. After reading, note the absorbance.
Serum antioxidant assessment
Using a capillary tube and chloroform anesthesia, blood was drawn via ocular puncture (Seotech Medical Laboratory Portharcourt, Nigeria). The blood samples were placed in plain-capped sample bottles, let to stand for two hours, and then centrifuged using a bucket centrifuge (B-Bran Scientific and Instrument Company, England) for five minutes at 1,000 rpm. For this experiment, serum was used as it settled to the top. Hydrogen peroxide was used as the substrate in the catalase (CAT) experiment to get the serum. Using published methods (19), reduced glutathione (GSH) was measured at 412 nm. Hydrogen peroxide was used as the substrate in the glutathione peroxidase (GPx) test (20). The procedure described elsewhere (20) was used to measure superoxide dismutase (SOD). The amount of malondialdehyde (MDA) in thiobarbituric acid reactive compounds (TBARS) was calculated as explained by previous studies (21,22). After that, 0.2 ml of 8.1% sodium dodecyl sulfate, 1.5 ml of 20% acetic acid solution adjusted to pH 3.5 using sodium hydroxide, and 1.5 ml of 0.8% thiobarbituric acid water solution was added to 0.2 ml of 10% (w/v) serum as a result of the mixed reaction. The mixture was raised to 4.0 ml using pure water and cooked for 60 minutes at 95°C. After adding about 1.0 mL of distilled water and 5.0 ml of the n-butanol and pyridine (15:1 v/v) mixture, the ice was centrifuged at 4000 rpm to cool it down. The absorbance was added after the data from MDA standards were summed at 532 nm, and the crude layer was eliminated. The concentrations were determined using absorption values as a reference for normal absorption.
Statistical analysis
All results are presented as mean ± SEM, n=4. One-way analysis of variance (ANOVA) was used to compare the differences within groups, and then post hoc multiple comparisons were performed. The analysis was conducted using the Excel analyzer and SPSS version 17.0 computer software. A significant threshold of p < 0.05 was established.
Results
Comparison of hematological indices in control and different experimental groups
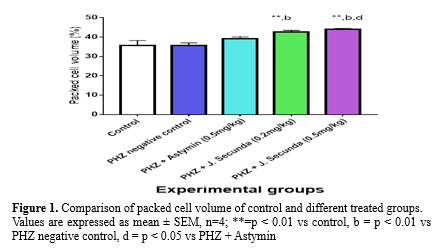
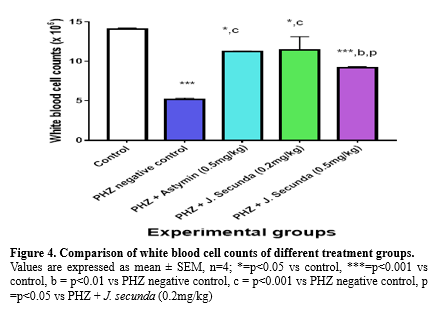
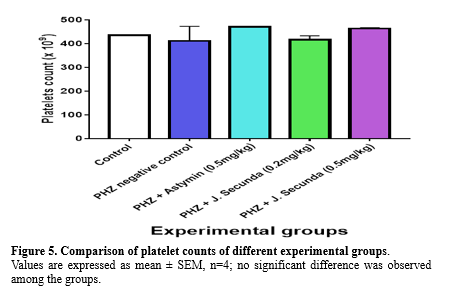
The mean concentration of PCV in PHZ + J. secunda (0.2mg/kg) and PHZ + J. secunda (0.5mg/kg) treated groups were significantly (p < 0.01) increased compared to control and PHZ negative control (Figure 1). However, PHZ + J. secunda- (0.5mg/kg) treated group significantly (p < 0.05) increased compared to PHZ+ Astymin (0.5mg/kg). Figure 2 below shows that the mean concentration of hemoglobin in the PHZ+ J. secunda (0.5mg/kg) treated group significantly (p < 0.01) increased compared to the control and PHZ negative control. Furthermore, PHZ + J. secunda- (0.2mg/kg) treated group significantly (p < 0.01) increased compared to PHZ negative control, and PHZ + J. secunda- (0.5mg/kg) treated group significantly (p < 0.01) increased compared to PHZ + astymin. Figure 3 shows that the mean RBC count in PHZ negative control significantly (p < 0.01) decreased compared to control. Furthermore, PHZ+ J. secunda (0.2mg/kg) and PHZ+ J. secunda (0.5mg/kg) treated groups significantly (p < 0.05) increased compared to control, PHZ negative control, and PHZ + Astymin. Figure 4 below shows the mean TWBC count in PHZ negative control, PHZ +Astymin (0.5 mg/kg), and PHZ + J. secunda significantly (p < 0.001) decreased compared to the controls. However, PHZ + Astymin (0.5 mg/kg) and PHZ + J. secunda treated groups significantly (p < 0.001) increased compared to PHZ negative controls. Furthermore, the PHZ+ J. secunda- (0.5mg/kg) treated group significantly (p < 0.001) decreased compared to the controls. Also, PHZ+ J. secunda (0.5mg/kg) significantly (p < 0.01) increased compared to PHZ negative control and significantly (p < 0.001) decreased compared to J. secunda (0.2mg/kg). Figure 5 demonstrates that the platelet counts in the treated groups had no significant statistical difference compared to the control.


Comparison of mean corpuscular hemoglobin concentration, mean corpuscular hemoglobin and mean corpuscular volume between control and experimental groups
The MCHC level in PHZ + astymin (0.5 mg/kg) considerably (p < 0.05) decreased in comparison to the control group, as shown in Table 2. In contrast to the PHZ negative control, the PHZ + astymin (0.5 mg/kg) and PHZ + J. secunda- (0.2 mg/kg) treated groups significantly (p < 0.05) decreased. Additionally, there was no statistically significant difference between the treated groups' mean corpuscular hemoglobin (MCH) and mean corpuscular volume (MCV) and the control (Table 2).
Comparison of mean differential white blood cell counts between control and experimental groups
The mean neutrophil count in PHZ+ astymin (0.5 mg/kg) considerably (p < 0.001) decreased in comparison to the control group, as shown in the table below. Additionally, there was a substantial (p < 0.05) drop in PHZ negative control and PHZ+ J. secunda (0.2 mg/kg) as compared to the control group. Furthermore, compared to PHZ+ astymin, there was a substantial (p < 0.05) increase in PHZ negative control and PHZ+ J. secunda (0.2 mg/kg). In comparison to the astymin group, there was a significant (p < 0.01) increase in PHZ+ J. secunda (0.5mg/kg). When comparing the treated groups to the control, there was no statistically significant difference in lymphocyte levels. The eosinophil count in the PHZ negative control increased significantly (p < 0.05) compared to the control. Additionally, compared to PHZ+ astymin (0.5mg/kg), PHZ+ J. secunda (0.5mg/kg) demonstrated a substantial (p < 0.05) increase. Furthermore, compared to the PHZ negative control, PHZ+ astymin (0.5 mg/kg) demonstrated a substantial (p < 0.01) decrease. The monocyte count rose significantly (p < 0.01) in comparison to the control in the PHZ negative control, PHZ plus J. secunda (0.2 mg/kg), and PHZ + J. secunda (0.5 mg/kg) groups. Additionally, there was a substantial rise in PHZ + J. secunda (0.2 mg/kg) and PHZ + J. secunda (0.5 mg/kg) as compared to PHZ + astymin (0.5 kg/kg). In addition, there was a substantial decrease in PHZ+ astymin (0.5 mg/kg) compared to the PHZ negative control (Table 3).
Comparison of serum total protein, albumin, bilirubin, and conjugate bilirubin concentration in control and different experimental groups
The mean serum total protein in PHZ + J. secunda (0.5 mg/kg) rose significantly (p < 0.05) compared to the control group, as shown in Table 4. When comparing the treated groups to the control, there was no statistically significant difference in albumin levels. The PHZ negative control, PHZ + astymin (0.5 mg/kg), and PHZ + J. secunda (0.2 mg/kg) all had mean bilirubin concentrations that were considerably (p < 0.001) higher than the control and significantly (p < 0.05) higher than the J. secunda control. Additionally, there was a substantial (p < 0.05) drop in the PHZ + J. secunda (0.5mg/kg) group compared to the control. Furthermore, compared to PHZ + astymin, PHZ + J. secunda (0.2 mg/kg) considerably (p < 0.01) decreased. Comparing PHZ + astymin to PHZ + J. secunda (0.5 mg/kg), there was a substantial (p < 0.001) decrease. When PHZ + J. secunda (0.5 mg/kg) was compared to J. secunda (0.2 mg/kg), there was a substantial (p < 0.05) decrease. In comparison to the control, conjugated bilirubin levels rose significantly (p < 0.001) in the PHZ negative control and PHZ+ astymin (0.5 mg/kg). Additionally, compared to the PHZ negative control, PHZ+ J. secunda (0.2 mg/kg) and PHZ + J. secunda (0.5 mg/kg) considerably (p < 0.001) decreased. Furthermore, in comparison to PHZ+ astymin, PHZ+ J. secunda (0.2 mg/kg) and PHZ + J. secunda (0.5 mg/kg) considerably (p < 0.001) reduced.
Concentration of serum urea and creatinine in control and different experimental groups
The mean blood creatinine concentration in the treated groups increased dramatically (p < 0.001) compared to the control, as Table 5 demonstrates. Additionally, there was a substantial (p < 0.001) rise in the PHZ + J. secunda (0.2 mg/kg) treated group compared to the negative control. Also, in comparison to the PHZ + astymin group, the PHZ+ J. secunda (0.2 mg/kg) group considerably (p < 0.001) rose, and compared to the PHZ + astymin group, the PHZ+ J. secunda (0.5 mg/kg) group significantly (p < 0.001) reduced. Furthermore, compared to PHZ + J. secunda (0.2 mg/kg), the PHZ+ J. secunda (0.5 mg/kg) group considerably (p < 0.001) decreased. Compared to the control group, the mean serum concentration of urea increased considerably (p < 0.001) in the treatment groups. Furthermore, there was a significant (p < 0.01) increase in the PHZ+ J. secunda (0.2 mg/kg) treated group when compared to the PHZ negative control and a significant (p < 0.01) decrease in the PHZ+ J. secunda (0.5 mg/kg) treated group was noted compared to the PHZ negative control. Additionally, there was a substantial (p < 0.01) rise in PHZ+ J. secunda (0.2 mg/kg) when compared to PHZ + astymin, and a significant (p < 0.01) decrease was observed in PHZ+ J. secunda (0.5 mg/kg) when compared to PHZ + Astymin.
The concentration of serum oxidative stress markers in the control and different experimental groups
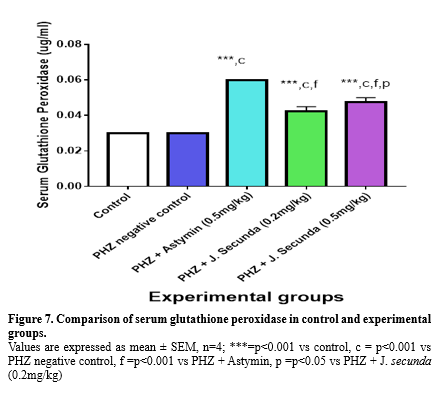
Figure 6 illustrates a significant (p < 0.001) increase in the mean serum glutathione concentration when comparing PHZ+ Astymin (0.5 mg/kg), PHZ+ J. secunda (0.2 mg/kg), and PHZ + J. secunda (0.5 mg/kg) to the control group. Additionally, in comparison to the PHZ negative control, the PHZ+ astymin (0.5 mg/kg), PHZ+ J. secunda (0.2 mg/kg), and PHZ + J. secunda (0.5 mg/kg) groups rose considerably (p<0.001). Additionally, compared to the PHZ + astymin (0.5 mg/kg) group, the PHZ + J. secunda (0.2 mg/kg) and PHZ + J. secunda (0.5 mg/kg) groups significantly (p < 0.001) decreased. Comparing PHZ + astymin (0.5 mg/kg) to PHZ + J. secunda (0.5 mg/kg), there was a substantial (p < 0.05) decrease. Comparing PHZ + J. secunda (0.5 mg/kg) to PHZ + J. secunda (0.2 mg/kg), there was a substantial (p < 0.05) increase. Serum glutathione peroxidase levels in the PHZ+ astymin (0.5 mg/kg), PHZ+ J. secunda (0.2 mg/kg), and PHZ + J. secunda (0.5 mg/kg) groups were considerably (p < 0.001) higher than those in the control group, as shown in Figure 7.
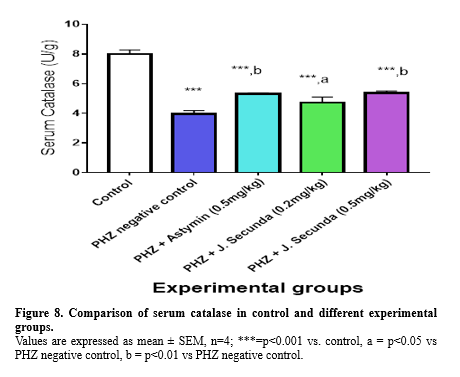
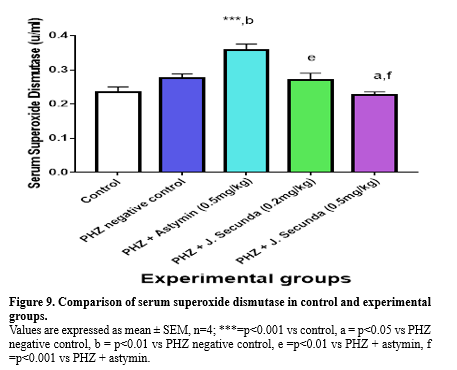
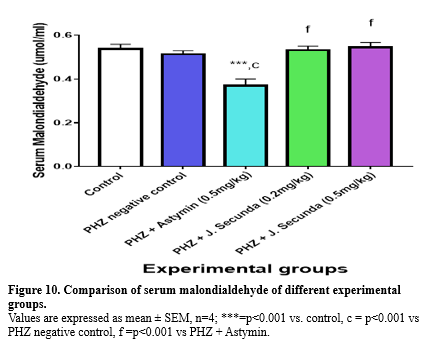
In addition, compared to the PHZ negative control, PHZ+ astymin (0.5 mg/kg), PHZ+ J. secunda (0.2 mg/kg), and PHZ + J. secunda (0.5 mg/kg) increased considerably (p < 0.001). Serum catalase was found to be considerably (p < 0.001) lower in the PHZ negative control, PHZ + astymin (0.5 mg/kg), PHZ + J. secunda (0.2 mg/kg), and PHZ + J. secunda (0.5 mg/kg) groups than in the control group (Figure 8). Additionally, compared to the PHZ negative control, the PHZ plus Astymin (0.5 mg/kg) and PHZ + J. secunda (0.5 mg/kg) groups rose significantly (p < 0.001). Furthermore, PHZ+ J. secunda (0.2 mg/kg) increased in comparison to the PHZ negative control significantly (p < 0.05). Figure 9 demonstrated a significant (p < 0.001) increase in serum superoxide dismutase in PHZ + astymin (0.5mg/kg) compared to the control group. In addition, PHZ + astymin (0.5 mg/kg) increased in comparison to the PHZ negative control significantly (p < 0.01). Furthermore, when comparing PHZ+ J. secunda (0.2 mg/kg) to PHZ+ astymin (0.5 mg/kg), there was a substantial (p<0.01) increase. When compared to the PHZ negative control, PHZ+ J. secunda (0.2 mg/kg) considerably (p < 0.05) decreased. When PHZ+ J. secunda (0.2 mg/kg) was compared to PHZ astymin (0.5 mg/kg), there was a substantial (p < 0.001) decrease. Figure 10 demonstrates a significant (p < 0.001) rise in serum malondialdehyde in the PHZ + astymin (0.5 mg/kg) group as compared to the control group. Additionally, when compared to the PHZ negative control, PHZ + astymin (0.5 mg/kg) considerably (p < 0.001) decreased. Furthermore, compared to PHZ + astymin (0.5 mg/kg), PHZ + J. secunda (0.2 mg/kg) and PHZ + J. secunda (0.5 mg/kg) rose considerably (p < 0.001).

Discussion
Pregnant women and young children are disproportionately affected by anemia, a major global health issue. According to statistics from the World Health Organization, globally, 40% of pregnant women and 42% of children under the age of five are anemic (1). There are various forms of anemia, many of which are uncommon, but they are all characterized by a decrease in the quantity of hemoglobin and red blood cells in the blood (23). Hemolytic anemia develops when red blood cells are lost more quickly than your bone marrow can produce them. Cancer, autoimmune diseases, and other illnesses may cause this. The cause determines the therapy (2). As a result, more people are using herbal treatments to treat their anemia. This study was conducted to discover how J. secunda and astymin affected the hematologic indices of anemic rats due to phenylhydrazine. Among the measures evaluated in this investigation are hematologic indices, serum urea and creatinine, total protein, albumin, total bilirubin, conjugated bilirubin, and serum oxidative stress indicators. It is possible that the hematologic enhancing potentials of J. secunda promoted erythropoietic growth factors required for erythropoiesis, which explains the considerable rise in PCV, hemoglobin, and red blood cells in J. secunda-treated rats as compared to control and phenylhydrazine negative control. Justicia secunda leaves have demonstrated hematinic, antibacterial, antisickling, and antihypertensive properties (13).
This study supported the previous (13) findings, which showed that rats fed with Justicia secunda had higher PCV, RBC, and hemoglobin levels. There was no significant increase in the platelet count in rats fed qurg Justicia secunda compared to the control. In anemia, both platelet counts and rate of platelet production increase significantly. An inverse relationship occurs with a fall in platelet count when anemia is controlled, as seen in rats fed with Justicia secunda. Moreover, some studies (24,14) have demonstrated that J. secunda has erythropoietic effects. Reduced antioxidant levels and the harmful effects of phenylhydrazine in this group may cause the notable drop in RBC count observed in the PHZ untreated group. The group treated with PHZ + astymin showed a substantial fall in the mean corpuscular hemoglobin concentration (MCHC), which could indicate hemoglobin-lowering anemia (25) observed a noteworthy decrease in the mean corpuscular hemoglobin concentration in rats with anemia produced by phenylhydrazine. The toxic effects of phenylhydrazine may have suppressed the white blood cells, as evidenced by the significant decrease in total white blood cells (TWBC) and altered differential WBC count in treated groups compared to the control. This effect was more pronounced in the phenylhydrazine negative control (Untreated). Virus susceptibility is the main sign of a compromised immune system (26). An individual with compromised immune function is more prone than the general population to infections, which may also be more severe or challenging to cure (26). The phagocytic qualities may account for the notable rise in monocyte concentration in PHZ negative control compared to the controls (27). White blood cells are essential to the body's defense system because they function as soldiers to keep the body safe against invasive pathogens (27). Furthermore, potentially harmful PHZ molecules in the blood may explain the notable increase in eosinophils observed in the PHZ-negative control group compared to the control group. Eosinophils are a crucial component of the body's defense system against parasites. Numerous eosinophils are produced during parasite infections, and these cells migrate to the tissues where the parasites are present (28). The cytokine IL-5 promotes the bone marrow's production of eosinophils and increases their survival in peripheral tissues, making them key players in most allergic reactions. Previous studies (29,30) reported a non-significant difference in the concentration of platelets in J. secunda-treated anemic rats, which corresponds with the result of this study.
This study indicated that the over-breakdown of red blood cells might have been the cause of the rise in total and conjugated bilirubin levels in the phenylhydrazine-treated rats. There is evidence that red blood cell destruction from phenylhydrazine exposure may lead to anemia and, ultimately, hyperbilirubinemia (31,32).
This study's substantial rise in serum urea and creatinine concentrations may indicate a potential renal injury due to phenylhydrazine administration. Renal failure may result from accumulating waste products from metabolism, such as creatinine and urea (27). The considerable drop in RBC, hemoglobin, and TWBC concentrations observed in the PHZ untreated group may be connected to the rise in creatinine and urea concentration, which may be a marker of renal toxicity. The production of erythropoietin, essential for erythropoiesis, occurs in the kidney (27). This supports the evidence (33) that a high creatinine concentration may be a sign of toxins that could cause renal failure.
The harmful effects of phenylhydrazine may have been the cause of the treatment groups' significantly lower serum catalase levels compared to the control group. This is consistent with the evidence in the literature (34), which states that oxidative stress caused by the medication in erythrocytes has long been linked to phenylhydrazine-induced toxicity. An imbalance between pro-oxidants and antioxidants is known as oxidative stress. It develops when the body's natural antioxidants are outweighed by the formation of reactive oxygen species (ROS) (35). The possible antioxidant qualities of astymin and J. secunda may be responsible for the notable increase in glutathione peroxidase, reduced glutathione, and superoxide dismutase observed in the astymin and J. secunda-treated groups relative to the control group. The harmful effect of p could cause the treatment groups' significantly lower serum catalase levels than the control group. Biomolecules known as antioxidants can stop or reduce the damage that free radicals do to cells. Enzymatic and non-enzymatic antioxidant defense mechanisms are categorized based on their ability to safeguard cellular constituents and maintain the cell's redox state (36). The increased quantities of flavonoids, phenols, and anthocyanins in the plant's leaf extract were suggested as the intense antioxidant scavenging activities in J. secunda leaf extract (37). Astymin is a specially prepared combination of vital minerals, vitamins, and amino acids (15). Exogenous antioxidants that mediate in-vivo activity include dietary antioxidants as vitamins, carotenoids, polyphenols, flavonoids, and bioflavonoids (15). Furthermore, the high antioxidant property of astymin may have prevented lipid peroxidation in this group, explaining the considerable drop in serum malondialdehyde in the astymin-treated group compared to the control. Membrane polyunsaturated fatty acid peroxidation produces MDA (38). Prostaglandin production is a process that also produces MDA (39).
Conclusion
Administration of phenylhydrazine, astymin, and Justicia secunda caused significant changes in hematologic indices, serum oxidative stress markers, urea, and creatinine. The treated group had higher antioxidant activities than the extract-treated group. This may be attributed to the composition of its amino acids and vitamins. Justicia secunda and astymin may possess erythropoietic potential. Phenylhydrazine caused an increase in oxidative stress, renal damage, and destruction of red, white, and platelet cells due to its toxic effects on blood cells. Based on the results of this study, we conclude that the combined use of J. secunda and astymin should be considered in the management of anemia. However, further study is required to ascertain how J. secunda mediates its erythropoietic effects.
Acknowledgement
The University of Calabar’s animal ethics committee approved our study protocol, for which the authors are grateful.
Funding sources
There was no funding assistance from any private or public sector.
Ethical statement
The University of Calabar's animal ethics committee permitted our research procedure with approval number 040PHY3719.
Conflicts of interest
There are no competing interests. Each author has read the document and given their consent for publication.
Author contributions
The study protocol was designed and written by M.S.K. M.S.K., C.B.G., U.L.I., and E.B.E., who conducted literature searches and lab tests. M.S.K. worked on data analysis and wrote the text. All authors read and approved the final manuscript.
According to the World Health Organization, globally, 40% of pregnant women and 42% of children under the age of five are anemic. Pregnant women and young children are disproportionately affected by anemia, a major global health issue (1). There are various forms of anemia, many of which are uncommon, but they are all characterized by a decrease in the quantity of hemoglobin and red blood cells in the blood (2). Hemolytic anemia develops when red blood cells are lost more quickly than the bone marrow can produce them. Cancer, autoimmune diseases, and other illnesses may be the cause, which determines the therapy (2). The body may produce acquired hemolytic anemia if it receives a signal to destroy healthy red blood cells (RBCs). Issues with the genes that regulate red blood cells are linked to inherited hemolytic anemia. Numerous internal and external factors can contribute to hemolytic anemia, including medicines related to the destruction of red blood cells. Hemolytic anemia is a feature of the clinical condition that is often associated with intoxication (3). Iron deficiency anemia is the most common single-nutrient deficiency disease (4). Iron deficiency may occur in increased demand, decreased intake, decreased or malabsorption, or chronic blood loss (5).
In 1895, Hermann Emil Fischer discovered the chemical compound phenylhydrazine, also known as hydroxybenzene. It is mainly used as a chemical intermediate in the pharmaceutical, agrochemical, and chemical industries (6). Once employed as antipyretics, phenylhydrazine and its derivatives have been shown to have toxic effects on blood cells; as a result, there is limited use of this compound as a medication. Instead, it is currently used as a chemical to induce anemia in animal studies (7). This results in a decrease in RBCs, HB, and hematocrit as well as an increase in the population of reticulocytes and the concentration of heme in serum.
In addition to being used as food, medicinal plants are the leading supplier of trade medicines. Despite advancements in the use of Western medications in healthcare delivery systems, medicinal plants remain an essential component of healthcare systems for both humans and animals. Approximately 80% of the world's population still depends on medicinal plants for basic medical requirements (8). Since medicinal plants have been found to possess bioactive molecules termed phytochemicals (9) and secondary metabolites that can protect humans against diseases (10), they have been reported as having therapeutic qualities utilized for the management of many disorders (10). It has been found that Justicia secunda works well for treating anemia (11). Justicia secunda is a member of the Acanthaceae family, also referred to as "sanguinaria" in Venezuela and "bloodroot" in Barbados. It is referred to as "obarabundu" locally in southeast Nigeria. It is known as "asindiri" or "ohowaazara" by the Ogbia people of Otuoke-Otuaba, Bayelsa, Niger-Delta region of Nigeria (12). J. secunda leaves are utilized in traditional medicine for wounds, anemia, and abdominal pain. It has been shown that the leaves of Justicia secunda exhibit hematinic, antisickling, antibacterial, and antihypertensive properties (13). The presence of tannins, flavonoids, alkaloids, quinines, anthocyanins, luteolin, anandamide, acetate, auranamide, quindoline, and pyrrolidone derivatives has been revealed by phytochemical screening of the plant (14).
A dietary supplement called astymin is crucial for children's growth, brain development, alertness, and overall health. Adults who use astymin report feeling less stressed about everyday life, having a stronger immune system, and recovering from illness more quickly (15). Astymin provides the body with essential amino acids needed to make protein, which helps build the body’s immune system. It also speeds up physical and mental growth in children, combats stress, quickens recovery from illness and allows the body to fight infection (15). This novel study aims to ascertain scientifically the hematopoietic potentials of Justicia secunda leaf in folklore medicine using phenylhydrazine-induced anemic Wistar rats.
Methods
Drugs and chemicals
The drugs and chemicals used included methanol, formalin, chloroform (Guangzhou JHD Chemical Reagent Co., Ltd. Shantou Guangdong, China), 2, 4-dinitrophenyl-hydrazine (Sigma-Aldrich Limited Germany), and astymin (Bayer Company, Inc., Leverkusen, Germany).
Hydro-methanol preparation of Justicia secunda
Fresh leaves of Justicia secunda were obtained and sent to a Botanist in the Faculty of Pharmacy, Madonna University for identification. They were dried at room temperature and ground to powder. For 48 hours, 590g of ground-up Justicia secunda was steeped in 1450 mL of methanol and 750 mL of water using an electronic weighing balance (Doran Scales, Inc., Batavia, Illinois, USA). The Whatman filter paper was used to sieve and filter the resulting suspension. Subsequently, the filtrate was concentrated in an electronic laboratory incubator (Labotech International Co., Ltd, Tokyo, Japan) at 60 °C. The jelly-like residue was introduced into an air-tight container and stored in the refrigerator until used. The LD50 of J. secunda was estimated to be 3800mg/kg body weight, as reported by previous studies (8). Hydromethanolic extraction was chosen to extract both hydrophilic and hydrophobic compounds of J. secunda.
Laboratory animals
For this study, we used twenty male Wistar rats weighing between 90 and 170g. The animals were housed in the Department of Physiology Animal House, University of Calabar, Nigeria. The animals were housed in standard animal cages (435 x 290 x 150 mm) with bedding made of wood shavings (5 rats per cage). They were subjected to a 12-hour light/dark cycle and given unlimited access to fresh water and feed (Provided by AEC Agrosystem Limited, Port Harcourt, and Rivers State, Nigeria). The National Committee for Research Ethics in Science and Technology (NENT), 2018, approved the guidelines for animal care, and they were acclimated to the conditions for seven days. The research protocol was approved by the University of Calabar's Animal Ethics Committee under approval number 040PHY3719.
Experimental design
The animals were randomly divided into 5 distinct groups (n = 4). At the end of seven days of acclimatization, 2, 4-dinitrophenyl-hydrazine, astymin, and J. secunda extract administration began. The treatment groups (B to E) received the drugs orally via gavage (Dosage per rat listed in Table 1) once a day, using the doses listed in Table 1. In contrast, the control group received feed and 0.5 ml of normal saline as a vehicle. Astymin and J. secunda extract administration lasted for 14 days, whereas 2, 4-dinitrophenyl-hydrazine was given intraperitoneal for just two days. Subsequently, the rats were anesthetized with chloroform, and blood samples were collected via ocular puncture. The samples were stored in an ice pack and immediately utilized to analyze hematologic parameters and serum oxidative stress markers.
|
Table 1. Study design and drugs administration
 |
Packed cell volume (PCV), total white blood cell count (TWBC), platelet count, and red cell count were the tested hematological parameters.
PCV: The ratio (L/L) representing the percentage of red blood cells in total blood is the packed cell volume. To measure PCV, ¾ of the capillary tube was filled with well-mixed EDTA blood. The empty end was sealed, the tube was placed in a microhematocrit rotor, and centrifugation was performed for five minutes. Using the microhematocrit reader, the PCV was read immediately following centrifugation. To calculate hemoglobin, the PCV number was divided by three HB units or mg/dl. The concentration of Hb in g/l in one liter of packed red blood cells was provided by the mean cell hemoglobin concentration (MCHC). MCHEg/L equals Hb. Red cell size is indicated by mean cell volume (MCV), which is expressed in femtolitres (Fl). The amount of hemoglobin in an average red cell picogram (pg) was calculated using mean cell hemoglobin (MCH). This technique had been applied previously (16).
Assay for RBC Count X1012/L
A procedure described in the literature (16) was used. Formal citrate (Diluting fluid) containing approximately 4.0 ml was measured and poured into a test tube. After mixing thoroughly, 0.02 milliliters of EDTA blood were added. After the counting chamber was put together and loaded with thoroughly blended samples, it was examined undisturbed using an x10 objective lens. The quantity of red cells per liter was determined by counting the cells in little squares.
RBC count = N x 201 X109. Where N=Number counted, 201 is the diluting factor, 0.2mm2=Area. 0.1mm = depth of the chamber.
Assay for total WBC count unit*109/L
The procedure described in the literature was followed (16). A test tube was filled with 0.38 ml of dilution fluid, then 0.02 ml of thoroughly mixed EDTA blood was added and stirred. The counting chamber was put together, and a Pasteur pipette was used to re-mix the diluted blood sample. The sample was placed into one of the chamber's grids. The white cells were allowed to settle in the compartment by remaining undisturbed for 20 mm. An X10 objective lens was used to inspect the sample. Four huge squares of the chamber were counted to determine the number of white cells per liter.
WBC count (Per liter) = N x Df X 106/A*D
N: number of cells counted, Df: Dilution factor, A: area counted, D: chamber depth.
Assay for platelet count unit *109/L
The procedure outlined in the literature (16) was followed. A test tube was filled with 0.38 ml of dilution fluid, then 0.02 ml of thoroughly mixed EDTA blood was added and stirred. The well-mixed sample was placed into the counting chamber after it had been built. The chamber was left undisturbed for 20mms. To prevent the fluid from drying, the chamber was placed in a Petri dish on dampened tissue and was covered with a lid.
An X10 objective lens was used for inspection, the platelets were counted in little squares, and the number of platelets per liter was noted.
Determination of serum urea
Reagent R1 (0.1 ml) was pipetted into each tube. An amount of 10 u of the sample, standard, and D/W was added into the tubes, mixed, and incubated at 37'c for 10 minutes. A volume of 2.5 mL of R2 and R3 was pipetted into each tube, mixed, and incubated at 25° C for 15 minutes. Subsequently, they were read and recorded the absorbance at 546 nm. This was done using the method described in the literature (17). Test tubes were labeled as test, standard, and blank.
Determination of serum creatinine
The procedure outlined in the literature was followed (17). Three labels were placed on the test, standard, and blank test tubes, and the reagent (2 mL) was pipetted into each tube. After adding 0.1 ml of each sample, standard, and D/W to the tubes and mixing, the absorbance of the sample and standard was measured after 30 seconds. The absorbance of the sample and standard was measured two minutes later-A2 for the sample and standard. A1-A2 equaled D.
Determination of serum total protein (Biuret method) unit g/l
The procedure outlined by the literature (18) was followed. The labels of the test tubes were blank, sample, and standard. One milliliter of the reagent was pipetted into each tube. The appropriate tube was filled with 0.02 ml of the standard, sample, and D/W, mixed, and incubated for 30 minutes at 25 °C. The blank, zero were utilized at 540 nm, then they were read and the absorption was recorded.
Determination of bilirubin (Jendrasik and Grof method) unit: Umol/L
The procedure outlined by previous studies was followed (18). Labels of the test tubes were blank, sample, and standard. Each tube was pipetted with 0.2 ml of the reagent, and then a drop of sodium nitrate was added to the tube labeled as the sample. The tubes were filled with 0.02 mL of sample and 1 mL of caffeine, stirred, and then incubated for 10 minutes at 25 °C. After mixing each tube with 1 ml of tartrate, the tubes were incubated at 25 °C for 5 to 30 minutes. The absorbance at 590 nm was set to zero.
Determination of serum albumin (Bromocresol Green method) unit g/L
The procedure outlined by previous studies (18) was followed. The labels of the test tubes were blank, sample, and standard. Three milliliters of the albumin reagent were pipetted into each tube. Intro-appropriate containers were filled with 0.01 mL of the sample, mixed, and incubated for 10 minutes at 25°C. The blank was used to zero the spectrophotometer at 578nm. After reading, note the absorbance.
Serum antioxidant assessment
Using a capillary tube and chloroform anesthesia, blood was drawn via ocular puncture (Seotech Medical Laboratory Portharcourt, Nigeria). The blood samples were placed in plain-capped sample bottles, let to stand for two hours, and then centrifuged using a bucket centrifuge (B-Bran Scientific and Instrument Company, England) for five minutes at 1,000 rpm. For this experiment, serum was used as it settled to the top. Hydrogen peroxide was used as the substrate in the catalase (CAT) experiment to get the serum. Using published methods (19), reduced glutathione (GSH) was measured at 412 nm. Hydrogen peroxide was used as the substrate in the glutathione peroxidase (GPx) test (20). The procedure described elsewhere (20) was used to measure superoxide dismutase (SOD). The amount of malondialdehyde (MDA) in thiobarbituric acid reactive compounds (TBARS) was calculated as explained by previous studies (21,22). After that, 0.2 ml of 8.1% sodium dodecyl sulfate, 1.5 ml of 20% acetic acid solution adjusted to pH 3.5 using sodium hydroxide, and 1.5 ml of 0.8% thiobarbituric acid water solution was added to 0.2 ml of 10% (w/v) serum as a result of the mixed reaction. The mixture was raised to 4.0 ml using pure water and cooked for 60 minutes at 95°C. After adding about 1.0 mL of distilled water and 5.0 ml of the n-butanol and pyridine (15:1 v/v) mixture, the ice was centrifuged at 4000 rpm to cool it down. The absorbance was added after the data from MDA standards were summed at 532 nm, and the crude layer was eliminated. The concentrations were determined using absorption values as a reference for normal absorption.
Statistical analysis
All results are presented as mean ± SEM, n=4. One-way analysis of variance (ANOVA) was used to compare the differences within groups, and then post hoc multiple comparisons were performed. The analysis was conducted using the Excel analyzer and SPSS version 17.0 computer software. A significant threshold of p < 0.05 was established.
Results
Comparison of hematological indices in control and different experimental groups
The mean concentration of PCV in PHZ + J. secunda (0.2mg/kg) and PHZ + J. secunda (0.5mg/kg) treated groups were significantly (p < 0.01) increased compared to control and PHZ negative control (Figure 1). However, PHZ + J. secunda- (0.5mg/kg) treated group significantly (p < 0.05) increased compared to PHZ+ Astymin (0.5mg/kg). Figure 2 below shows that the mean concentration of hemoglobin in the PHZ+ J. secunda (0.5mg/kg) treated group significantly (p < 0.01) increased compared to the control and PHZ negative control. Furthermore, PHZ + J. secunda- (0.2mg/kg) treated group significantly (p < 0.01) increased compared to PHZ negative control, and PHZ + J. secunda- (0.5mg/kg) treated group significantly (p < 0.01) increased compared to PHZ + astymin. Figure 3 shows that the mean RBC count in PHZ negative control significantly (p < 0.01) decreased compared to control. Furthermore, PHZ+ J. secunda (0.2mg/kg) and PHZ+ J. secunda (0.5mg/kg) treated groups significantly (p < 0.05) increased compared to control, PHZ negative control, and PHZ + Astymin. Figure 4 below shows the mean TWBC count in PHZ negative control, PHZ +Astymin (0.5 mg/kg), and PHZ + J. secunda significantly (p < 0.001) decreased compared to the controls. However, PHZ + Astymin (0.5 mg/kg) and PHZ + J. secunda treated groups significantly (p < 0.001) increased compared to PHZ negative controls. Furthermore, the PHZ+ J. secunda- (0.5mg/kg) treated group significantly (p < 0.001) decreased compared to the controls. Also, PHZ+ J. secunda (0.5mg/kg) significantly (p < 0.01) increased compared to PHZ negative control and significantly (p < 0.001) decreased compared to J. secunda (0.2mg/kg). Figure 5 demonstrates that the platelet counts in the treated groups had no significant statistical difference compared to the control.





Comparison of mean corpuscular hemoglobin concentration, mean corpuscular hemoglobin and mean corpuscular volume between control and experimental groups
The MCHC level in PHZ + astymin (0.5 mg/kg) considerably (p < 0.05) decreased in comparison to the control group, as shown in Table 2. In contrast to the PHZ negative control, the PHZ + astymin (0.5 mg/kg) and PHZ + J. secunda- (0.2 mg/kg) treated groups significantly (p < 0.05) decreased. Additionally, there was no statistically significant difference between the treated groups' mean corpuscular hemoglobin (MCH) and mean corpuscular volume (MCV) and the control (Table 2).
Comparison of mean differential white blood cell counts between control and experimental groups
The mean neutrophil count in PHZ+ astymin (0.5 mg/kg) considerably (p < 0.001) decreased in comparison to the control group, as shown in the table below. Additionally, there was a substantial (p < 0.05) drop in PHZ negative control and PHZ+ J. secunda (0.2 mg/kg) as compared to the control group. Furthermore, compared to PHZ+ astymin, there was a substantial (p < 0.05) increase in PHZ negative control and PHZ+ J. secunda (0.2 mg/kg). In comparison to the astymin group, there was a significant (p < 0.01) increase in PHZ+ J. secunda (0.5mg/kg). When comparing the treated groups to the control, there was no statistically significant difference in lymphocyte levels. The eosinophil count in the PHZ negative control increased significantly (p < 0.05) compared to the control. Additionally, compared to PHZ+ astymin (0.5mg/kg), PHZ+ J. secunda (0.5mg/kg) demonstrated a substantial (p < 0.05) increase. Furthermore, compared to the PHZ negative control, PHZ+ astymin (0.5 mg/kg) demonstrated a substantial (p < 0.01) decrease. The monocyte count rose significantly (p < 0.01) in comparison to the control in the PHZ negative control, PHZ plus J. secunda (0.2 mg/kg), and PHZ + J. secunda (0.5 mg/kg) groups. Additionally, there was a substantial rise in PHZ + J. secunda (0.2 mg/kg) and PHZ + J. secunda (0.5 mg/kg) as compared to PHZ + astymin (0.5 kg/kg). In addition, there was a substantial decrease in PHZ+ astymin (0.5 mg/kg) compared to the PHZ negative control (Table 3).
Comparison of serum total protein, albumin, bilirubin, and conjugate bilirubin concentration in control and different experimental groups
The mean serum total protein in PHZ + J. secunda (0.5 mg/kg) rose significantly (p < 0.05) compared to the control group, as shown in Table 4. When comparing the treated groups to the control, there was no statistically significant difference in albumin levels. The PHZ negative control, PHZ + astymin (0.5 mg/kg), and PHZ + J. secunda (0.2 mg/kg) all had mean bilirubin concentrations that were considerably (p < 0.001) higher than the control and significantly (p < 0.05) higher than the J. secunda control. Additionally, there was a substantial (p < 0.05) drop in the PHZ + J. secunda (0.5mg/kg) group compared to the control. Furthermore, compared to PHZ + astymin, PHZ + J. secunda (0.2 mg/kg) considerably (p < 0.01) decreased. Comparing PHZ + astymin to PHZ + J. secunda (0.5 mg/kg), there was a substantial (p < 0.001) decrease. When PHZ + J. secunda (0.5 mg/kg) was compared to J. secunda (0.2 mg/kg), there was a substantial (p < 0.05) decrease. In comparison to the control, conjugated bilirubin levels rose significantly (p < 0.001) in the PHZ negative control and PHZ+ astymin (0.5 mg/kg). Additionally, compared to the PHZ negative control, PHZ+ J. secunda (0.2 mg/kg) and PHZ + J. secunda (0.5 mg/kg) considerably (p < 0.001) decreased. Furthermore, in comparison to PHZ+ astymin, PHZ+ J. secunda (0.2 mg/kg) and PHZ + J. secunda (0.5 mg/kg) considerably (p < 0.001) reduced.
Concentration of serum urea and creatinine in control and different experimental groups
The mean blood creatinine concentration in the treated groups increased dramatically (p < 0.001) compared to the control, as Table 5 demonstrates. Additionally, there was a substantial (p < 0.001) rise in the PHZ + J. secunda (0.2 mg/kg) treated group compared to the negative control. Also, in comparison to the PHZ + astymin group, the PHZ+ J. secunda (0.2 mg/kg) group considerably (p < 0.001) rose, and compared to the PHZ + astymin group, the PHZ+ J. secunda (0.5 mg/kg) group significantly (p < 0.001) reduced. Furthermore, compared to PHZ + J. secunda (0.2 mg/kg), the PHZ+ J. secunda (0.5 mg/kg) group considerably (p < 0.001) decreased. Compared to the control group, the mean serum concentration of urea increased considerably (p < 0.001) in the treatment groups. Furthermore, there was a significant (p < 0.01) increase in the PHZ+ J. secunda (0.2 mg/kg) treated group when compared to the PHZ negative control and a significant (p < 0.01) decrease in the PHZ+ J. secunda (0.5 mg/kg) treated group was noted compared to the PHZ negative control. Additionally, there was a substantial (p < 0.01) rise in PHZ+ J. secunda (0.2 mg/kg) when compared to PHZ + astymin, and a significant (p < 0.01) decrease was observed in PHZ+ J. secunda (0.5 mg/kg) when compared to PHZ + Astymin.
The concentration of serum oxidative stress markers in the control and different experimental groups
Figure 6 illustrates a significant (p < 0.001) increase in the mean serum glutathione concentration when comparing PHZ+ Astymin (0.5 mg/kg), PHZ+ J. secunda (0.2 mg/kg), and PHZ + J. secunda (0.5 mg/kg) to the control group. Additionally, in comparison to the PHZ negative control, the PHZ+ astymin (0.5 mg/kg), PHZ+ J. secunda (0.2 mg/kg), and PHZ + J. secunda (0.5 mg/kg) groups rose considerably (p<0.001). Additionally, compared to the PHZ + astymin (0.5 mg/kg) group, the PHZ + J. secunda (0.2 mg/kg) and PHZ + J. secunda (0.5 mg/kg) groups significantly (p < 0.001) decreased. Comparing PHZ + astymin (0.5 mg/kg) to PHZ + J. secunda (0.5 mg/kg), there was a substantial (p < 0.05) decrease. Comparing PHZ + J. secunda (0.5 mg/kg) to PHZ + J. secunda (0.2 mg/kg), there was a substantial (p < 0.05) increase. Serum glutathione peroxidase levels in the PHZ+ astymin (0.5 mg/kg), PHZ+ J. secunda (0.2 mg/kg), and PHZ + J. secunda (0.5 mg/kg) groups were considerably (p < 0.001) higher than those in the control group, as shown in Figure 7.
In addition, compared to the PHZ negative control, PHZ+ astymin (0.5 mg/kg), PHZ+ J. secunda (0.2 mg/kg), and PHZ + J. secunda (0.5 mg/kg) increased considerably (p < 0.001). Serum catalase was found to be considerably (p < 0.001) lower in the PHZ negative control, PHZ + astymin (0.5 mg/kg), PHZ + J. secunda (0.2 mg/kg), and PHZ + J. secunda (0.5 mg/kg) groups than in the control group (Figure 8). Additionally, compared to the PHZ negative control, the PHZ plus Astymin (0.5 mg/kg) and PHZ + J. secunda (0.5 mg/kg) groups rose significantly (p < 0.001). Furthermore, PHZ+ J. secunda (0.2 mg/kg) increased in comparison to the PHZ negative control significantly (p < 0.05). Figure 9 demonstrated a significant (p < 0.001) increase in serum superoxide dismutase in PHZ + astymin (0.5mg/kg) compared to the control group. In addition, PHZ + astymin (0.5 mg/kg) increased in comparison to the PHZ negative control significantly (p < 0.01). Furthermore, when comparing PHZ+ J. secunda (0.2 mg/kg) to PHZ+ astymin (0.5 mg/kg), there was a substantial (p<0.01) increase. When compared to the PHZ negative control, PHZ+ J. secunda (0.2 mg/kg) considerably (p < 0.05) decreased. When PHZ+ J. secunda (0.2 mg/kg) was compared to PHZ astymin (0.5 mg/kg), there was a substantial (p < 0.001) decrease. Figure 10 demonstrates a significant (p < 0.001) rise in serum malondialdehyde in the PHZ + astymin (0.5 mg/kg) group as compared to the control group. Additionally, when compared to the PHZ negative control, PHZ + astymin (0.5 mg/kg) considerably (p < 0.001) decreased. Furthermore, compared to PHZ + astymin (0.5 mg/kg), PHZ + J. secunda (0.2 mg/kg) and PHZ + J. secunda (0.5 mg/kg) rose considerably (p < 0.001).
Table 2. Comparison of mean corpuscular hemoglobin concentration, mean corpuscular hemoglobin, and mean corpuscular volume between control and experimental groups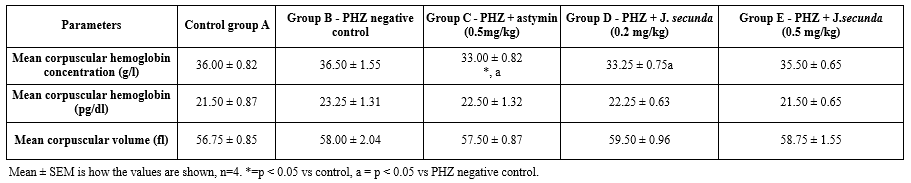 Table 3. Comparison of mean differential white blood cell counts between control and experimental groups 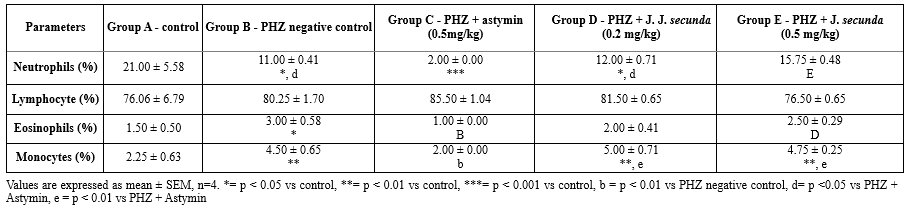 Table 4. Comparison of serum total protein, albumin, bilirubin, and conjugate bilirubin concentration in the control and treatment groups 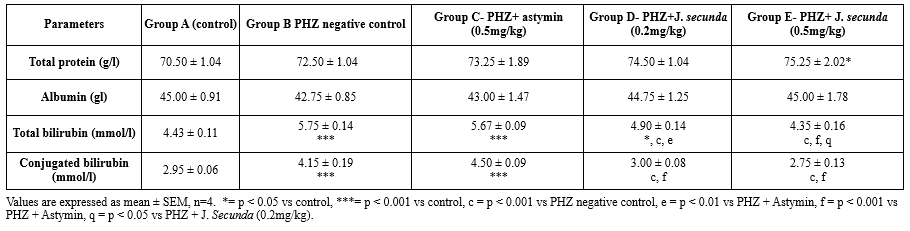 |
Table 5. Comparison of serum urea and creatinine concentration in control and different experimental groups |





Discussion
Pregnant women and young children are disproportionately affected by anemia, a major global health issue. According to statistics from the World Health Organization, globally, 40% of pregnant women and 42% of children under the age of five are anemic (1). There are various forms of anemia, many of which are uncommon, but they are all characterized by a decrease in the quantity of hemoglobin and red blood cells in the blood (23). Hemolytic anemia develops when red blood cells are lost more quickly than your bone marrow can produce them. Cancer, autoimmune diseases, and other illnesses may cause this. The cause determines the therapy (2). As a result, more people are using herbal treatments to treat their anemia. This study was conducted to discover how J. secunda and astymin affected the hematologic indices of anemic rats due to phenylhydrazine. Among the measures evaluated in this investigation are hematologic indices, serum urea and creatinine, total protein, albumin, total bilirubin, conjugated bilirubin, and serum oxidative stress indicators. It is possible that the hematologic enhancing potentials of J. secunda promoted erythropoietic growth factors required for erythropoiesis, which explains the considerable rise in PCV, hemoglobin, and red blood cells in J. secunda-treated rats as compared to control and phenylhydrazine negative control. Justicia secunda leaves have demonstrated hematinic, antibacterial, antisickling, and antihypertensive properties (13).
This study supported the previous (13) findings, which showed that rats fed with Justicia secunda had higher PCV, RBC, and hemoglobin levels. There was no significant increase in the platelet count in rats fed qurg Justicia secunda compared to the control. In anemia, both platelet counts and rate of platelet production increase significantly. An inverse relationship occurs with a fall in platelet count when anemia is controlled, as seen in rats fed with Justicia secunda. Moreover, some studies (24,14) have demonstrated that J. secunda has erythropoietic effects. Reduced antioxidant levels and the harmful effects of phenylhydrazine in this group may cause the notable drop in RBC count observed in the PHZ untreated group. The group treated with PHZ + astymin showed a substantial fall in the mean corpuscular hemoglobin concentration (MCHC), which could indicate hemoglobin-lowering anemia (25) observed a noteworthy decrease in the mean corpuscular hemoglobin concentration in rats with anemia produced by phenylhydrazine. The toxic effects of phenylhydrazine may have suppressed the white blood cells, as evidenced by the significant decrease in total white blood cells (TWBC) and altered differential WBC count in treated groups compared to the control. This effect was more pronounced in the phenylhydrazine negative control (Untreated). Virus susceptibility is the main sign of a compromised immune system (26). An individual with compromised immune function is more prone than the general population to infections, which may also be more severe or challenging to cure (26). The phagocytic qualities may account for the notable rise in monocyte concentration in PHZ negative control compared to the controls (27). White blood cells are essential to the body's defense system because they function as soldiers to keep the body safe against invasive pathogens (27). Furthermore, potentially harmful PHZ molecules in the blood may explain the notable increase in eosinophils observed in the PHZ-negative control group compared to the control group. Eosinophils are a crucial component of the body's defense system against parasites. Numerous eosinophils are produced during parasite infections, and these cells migrate to the tissues where the parasites are present (28). The cytokine IL-5 promotes the bone marrow's production of eosinophils and increases their survival in peripheral tissues, making them key players in most allergic reactions. Previous studies (29,30) reported a non-significant difference in the concentration of platelets in J. secunda-treated anemic rats, which corresponds with the result of this study.
This study indicated that the over-breakdown of red blood cells might have been the cause of the rise in total and conjugated bilirubin levels in the phenylhydrazine-treated rats. There is evidence that red blood cell destruction from phenylhydrazine exposure may lead to anemia and, ultimately, hyperbilirubinemia (31,32).
This study's substantial rise in serum urea and creatinine concentrations may indicate a potential renal injury due to phenylhydrazine administration. Renal failure may result from accumulating waste products from metabolism, such as creatinine and urea (27). The considerable drop in RBC, hemoglobin, and TWBC concentrations observed in the PHZ untreated group may be connected to the rise in creatinine and urea concentration, which may be a marker of renal toxicity. The production of erythropoietin, essential for erythropoiesis, occurs in the kidney (27). This supports the evidence (33) that a high creatinine concentration may be a sign of toxins that could cause renal failure.
The harmful effects of phenylhydrazine may have been the cause of the treatment groups' significantly lower serum catalase levels compared to the control group. This is consistent with the evidence in the literature (34), which states that oxidative stress caused by the medication in erythrocytes has long been linked to phenylhydrazine-induced toxicity. An imbalance between pro-oxidants and antioxidants is known as oxidative stress. It develops when the body's natural antioxidants are outweighed by the formation of reactive oxygen species (ROS) (35). The possible antioxidant qualities of astymin and J. secunda may be responsible for the notable increase in glutathione peroxidase, reduced glutathione, and superoxide dismutase observed in the astymin and J. secunda-treated groups relative to the control group. The harmful effect of p could cause the treatment groups' significantly lower serum catalase levels than the control group. Biomolecules known as antioxidants can stop or reduce the damage that free radicals do to cells. Enzymatic and non-enzymatic antioxidant defense mechanisms are categorized based on their ability to safeguard cellular constituents and maintain the cell's redox state (36). The increased quantities of flavonoids, phenols, and anthocyanins in the plant's leaf extract were suggested as the intense antioxidant scavenging activities in J. secunda leaf extract (37). Astymin is a specially prepared combination of vital minerals, vitamins, and amino acids (15). Exogenous antioxidants that mediate in-vivo activity include dietary antioxidants as vitamins, carotenoids, polyphenols, flavonoids, and bioflavonoids (15). Furthermore, the high antioxidant property of astymin may have prevented lipid peroxidation in this group, explaining the considerable drop in serum malondialdehyde in the astymin-treated group compared to the control. Membrane polyunsaturated fatty acid peroxidation produces MDA (38). Prostaglandin production is a process that also produces MDA (39).
Conclusion
Administration of phenylhydrazine, astymin, and Justicia secunda caused significant changes in hematologic indices, serum oxidative stress markers, urea, and creatinine. The treated group had higher antioxidant activities than the extract-treated group. This may be attributed to the composition of its amino acids and vitamins. Justicia secunda and astymin may possess erythropoietic potential. Phenylhydrazine caused an increase in oxidative stress, renal damage, and destruction of red, white, and platelet cells due to its toxic effects on blood cells. Based on the results of this study, we conclude that the combined use of J. secunda and astymin should be considered in the management of anemia. However, further study is required to ascertain how J. secunda mediates its erythropoietic effects.
Acknowledgement
The University of Calabar’s animal ethics committee approved our study protocol, for which the authors are grateful.
Funding sources
There was no funding assistance from any private or public sector.
Ethical statement
The University of Calabar's animal ethics committee permitted our research procedure with approval number 040PHY3719.
Conflicts of interest
There are no competing interests. Each author has read the document and given their consent for publication.
Author contributions
The study protocol was designed and written by M.S.K. M.S.K., C.B.G., U.L.I., and E.B.E., who conducted literature searches and lab tests. M.S.K. worked on data analysis and wrote the text. All authors read and approved the final manuscript.
Article Type: Research |
Subject:
Basic medical sciences
References
1. McLean E, Cogswell M, Egli I, Wojdyla D, De-Benoist B. Worldwide prevalence of anemia, WHO vitamin and mineral nutrition information system, 1993-2005. Public Health Nutr. 2009;12(4):444-54. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Finbarrs BE, Christian EM. and Anikwe VA. Hematological and Histological Effects of Ficus Sycomorus Leaf Extract on Bone Marrow of Phenylhdrazine-Induced Haemolytic Anaemia. J Bio Innov. 2020;9(5):1056-72. [View at Publisher] [DOI] [Google Scholar]
3. Beutler E, Grabowski GA. Gaucher disease: The Metabolic and Molecular Bases of Inherited Disease. 2001:3635-68. [View at Publisher] [Google Scholar]
4. Black MM, Quigg AM, Hurley KM, Pepper MR. Iron deficiency and iron-deficiency anemia in the first two years of life: strategies to prevent loss of developmental potential. Nutr Rev. 2011;69(Suppl1):S64-S70. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Cappellini MD, Musallam KM, Taher AT. Iron deficiency anaemia revisited. J Intern Med. 2020;287(2):153-70. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Pandey K, Meena AK, Jain A, Singh RK. Molecular Mechanism of Phenylhydrazine Induced Haematotoxicity: Ame J Phytomed Clin Therapeut. 2014;2(3):390-4. [View at Publisher] [Google Scholar]
7. Hosseini A, Hosseinzadeh H. Effect of Nigella sativa on Blood Diseases: A Review. (PR Victor, RW Ronald, Eds.). Nuts and Seeds in Health and Disease Prevention (2nd Ed.). 2020;315-28. [View at Publisher] [DOI] [Google Scholar]
8. Onochie AU, Helen Oli A, Nnamdi Oli A, Ezeigwe OC, Nwaka AC, Okani CO, et al. The Pharmacobiochemical Effects of Ethanol Extract of Justicia secunda Vahl Leaves in Rattus Norvegicus. J Exp Pharmacol. 2020;12:423-37. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Fasuyi AO. Nutritional potentials of some tropical vegetable leaf meals: chemical characterization and functional properties. African Journal of Biotechnology. 2006;5(1):49-53. [View at Publisher] [Google Scholar]
10. Sharma AK,Sharma A. Plant secondary metabolites: physicochemical properties and therapeutic applications. Springer. 2022; 371-413. [View at Publisher] [DOI] [Google Scholar]
11. Onyeabo C, Achi NK, Ekeleme-Egedigwe CA, Ebere CU, Okoro CK. Hematological and Biochemical Studies on Justicia Carnea Leaves Extract in Phenylhydrazine Induced-Anemia in Albino Rats. Acta Sci Pol Technol Aliment. 2017;16(2):217-30. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Umoh RA, Johnny II, Umoh OT, Udoh AE, Anah VU, Obah-Eni LC. Pharmacognostic Evaluation of the Leaves and Stems of Justicia Secunda Vahl. World Journal of Pharmaceutical Research. 2020;9(11):5-18. [View at Publisher] [Google Scholar]
13. Anyasor NG, Azeezat Adenike OA. and Babafemi O. (2019). Evaluation of the anti-inflammatory activity of Justicia secunda Vahl leaf extract using in vitro and in vivo inflammation models. Clinical phytoscience. 2019;5(49):1-13. [View at Publisher] [DOI] [Google Scholar]
14. Onoja SO, Ezeja MI, Omeh YN, Onwukwe BC. Antioxidant, anti-inflammatory, and antinociceptive activities of methanolic extract of Justicia secunda leaves. Alexandria Journal of Medicine. 2017;53(3):207-13. [View at Publisher] [DOI] [Google Scholar]
15. Nneoyi-Egbe AF, Onyenweaku E, Akpanukoh A, Ebai P. Haematinic and hepatoprotective properties of Telfairia occidentalis fruit mesocarp on phenylhydrazine-induced anemia in experimental rats. Biochem Res Int. 2023;2023:8838481. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Obembe AO, Omini GC, Okon UA, Okpo-ene AI, Ikpi DE. Hematological and immunological effect of Cannabis sativa on Albino Wistar rats. Br J Med Med Res. 2015;7(1):52-60. [View at Publisher] [DOI] [Google Scholar]
17. Mobisson SK, Onyebuagu PC, Wopara I, Woha BJ, Boma HO, Abaka OS, et al. Cannabidiol Oil and Prednisolone Treatment Altered Hematologic Indices, Serum Urea, Creatinine and Cellular Architecture of Kidney on Cadmium Induced Toxicity in Male Wistar Rats. J Pharm Res Int. 2023;35(21):14-27. [View at Publisher] [DOI] [Google Scholar]
18. Ukoh IE, Umoh IB, Ukpai EE, Mobisson SK, Whiskey IP, Azosibe P, et al. Thermoxidized palm oil diet (TPO) induced protein dearangements nin rats is ameliorated by fresh palm oil (FPO) and vitamin E. Journal of Phytopharmacology. 2024;13(2):154-9. [View at Publisher] [DOI] [Google Scholar]
19. Luchese C, Pinton S, Nogueira CW. Brain and lungs of rats are differently affected by cigarette smoke exposure: Antioxidant effect of an organoselenium compound. Pharmacol Res. 2009;59(3):194-201. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Meenakshi C, Umesh KJ, Mohammed AK, Sunaina Z, Tasneem F. Effect of heavy metal stress on proline, malondialdehyde and superoxide dismutase activity in the cyanobacterium spirulina platensis-S5. Ecotoxicol Environ Saf. 2007;66(2):204-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by the thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Walker R, Whittlesea C, Eds. Clinical pharmacy and therapeutic. 5th ed. New York:Churchill Livingstone;2007. p.699-701. [View at Publisher]
24. Mpiana PT, Ngbolua KN, Bokota MT, Kasonga TK, Atibu EK, Tshibangu DS, et al. In vitro effects of anthocyanin extracts from Justicia secunda Vahl on the solubility of hemoglobin S and membrane stability of sickle erythrocytes. Blood Transfus. 2010;8(4):248-54. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Shukla P, Singh RK. Toxicogenomics of Phenylhydrazine Induced Hematotoxicity and its Attenuation by Plumbagin from Plumbago zeylanica. Pharmacogn Mag. 2015;11(Suppl 3):S380-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Mozafari H, Pourpak Z, Pourseyed R, Moein M, Entezari N, Farhadi A, Aghamohammadi A, et al. Health-related quality of life in primary immune deficient patients. Iran J Allergy Asthma Immunol. 2006;5(1):23-7. [View at Publisher] [PMID] [Google Scholar]
27. Guyton AC, Hall JE. Textbook of Medical Physiology. 11th Ed. Philadelphia:Elsevier Saunders;2012. p.802- 4. [View at Publisher] [Google Scholar]
28. Cadman ET, Thysse KA, Bearder S, Cheung AYN, Johnston AC, Lee JJ, et al. Eosinophils Are Important for Protection, Immunoregulation, and Pathology during Infection with Nematode Microfilariae. PloS Pathog. 2014;10(3):e1003988. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Chaplin DD. Overview of the Immune Response. J Allergy Clin Immunol. 2010;125(Suppl2):S3-23. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Irinmwinuwa EO, Afonne JO. Investigation into the effect of ethanol leaf extract and fractions of Justicia Secunda on platelets and WBC counts in mice. Magna Scientia Advanced Research and Reviews. 2022;6(1):1-7. [View at Publisher] [DOI] [Google Scholar]
31. Cary R, Dobson S. and Brooke I. Phenylhydrazine. Concise International Chemical Assessment Document 19. 2000:6-27. [View at Publisher]
32. Khatheeja S, Prabhakaran AR, Safiullah A. FTIR-ATR Studies on Phenylhydrazine Induced Hyperbilirubinemia in Wistar Rat. Int J Health Sci Res. 2018;8(2):212-20 [View at Publisher] [Google Scholar]
33. Paul R, Minay J, Christopher C, Damian F, Kelly C. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-31. [View at Publisher] [DOI] [PMID] [Google Scholar]
34. Jain SK, Hochstein P. Membrane alterations in phenylhydrazine-induced reticulocytes. Arch Biochem Biophys. 1980;201(2):683-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
35. Tejchman K, Kotfis K, Sieńko J. Biomarkers and Mechanisms of Oxidative Stress-Last 20 Years of Research with an Emphasis on Kidney Damage and Renal Transplantation. Int J Mol Sci. 2021;22(15):8010. [View at Publisher] [DOI] [PMID] [Google Scholar]
36. Dalvi U, Naik R, Kale A. Antioxidative enzyme responses against fusarium wilt (fusarium oxysporum f. sp. Ciceris) in chickpea genotypes. Annu Res Rev Biol. 2017;12(5):1-9. [View at Publisher] [DOI] [Google Scholar]
37. Hamilton- Amachree A, Osioma E. Comparative Study on the Phytochemical and in vitro Antioxidant Properties of Methanolic Leaf Extract of Justicia Secunda Vahl. Nigerian Journal of Science and Environment. 2017;15(1):111-7. [View at Publisher] [DOI] [Google Scholar]
38. Mobisson SK, Ikpi DE, Wopara I, Obembe AO. Cannabis sativa exacerbates testicular function by increased oxidative stress, altered male reproductive hormones, sperm quality/quantity, and cellular architecture of the testis. Andrologia. 2022;54(9):e14492. [View at Publisher] [DOI] [PMID] [Google Scholar]
39. Marnett LJ. Oxy radicals, lipid peroxidation, and DNA damage. Toxicology. 2002;181-182:219-22. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



