Volume 5, Issue 3 ( Journal of Clinical and Basic Research (JCBR) 2021)
jcbr 2021, 5(3): 33-39 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ebrahimi M, Askari F S, Razzaghi N, Mohebbi A. SARS-Cov-2 and Mycobacterium tuberculosis: A Mini Review. jcbr 2021; 5 (3) :33-39
URL: http://jcbr.goums.ac.ir/article-1-319-en.html
URL: http://jcbr.goums.ac.ir/article-1-319-en.html
1- Children's Research Center, Golestan University of Medical Sciences, Gorgan, Iran
2- Department of Microbiology, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
3- Vista Aria Rena Gene Inc/Department of Microbiology, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran , mohebbi-a@goums.ac.ir
2- Department of Microbiology, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
3- Vista Aria Rena Gene Inc/Department of Microbiology, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran , mohebbi-a@goums.ac.ir
Full-Text [PDF 384 kb]
(550 Downloads)
| Abstract (HTML) (1963 Views)
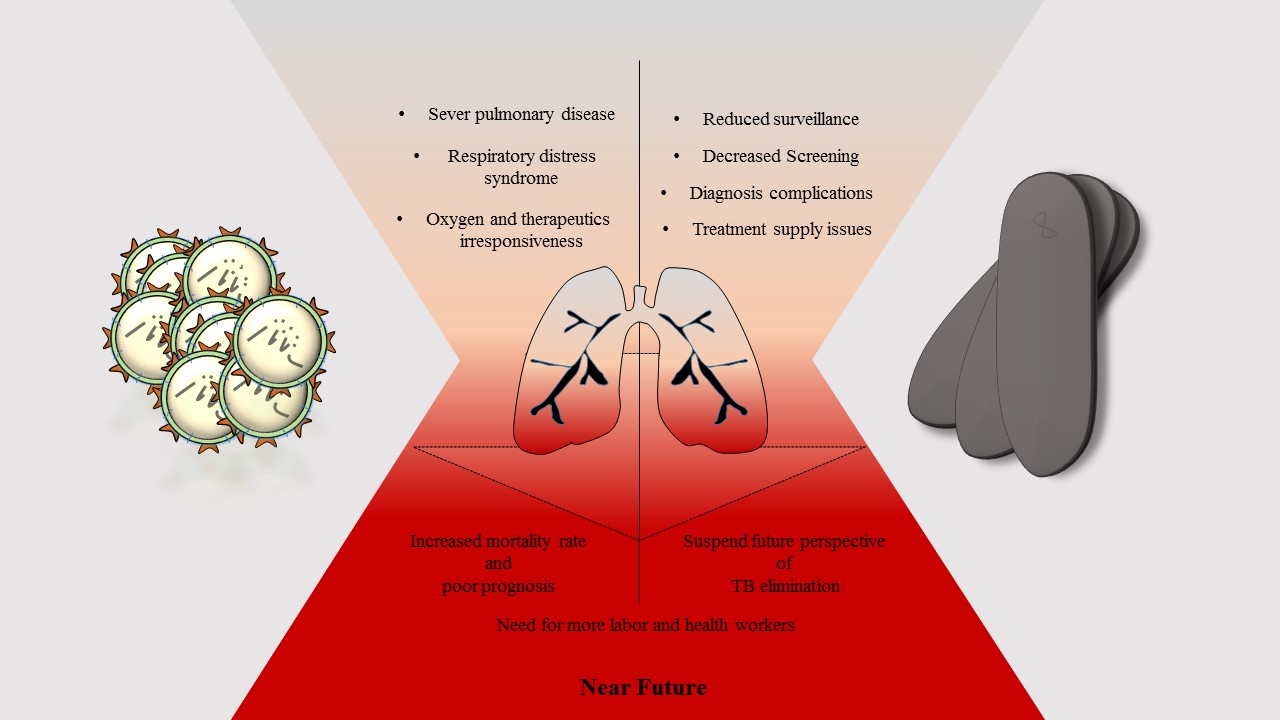

Figure 1. Schematic representation of crosstalk between TB and COVID-19. The future of TB elimination and better COVID-19 prognosis are closely interlinked
We have also suggested the assessment of the efficiency of COVID-19 vaccination in patients co-infected with TB. Until the mass vaccination program, good prognosis practices need to be implemented to minimize transmission and mortality of SARS-CoV-2.
CONCLUSION
Managing interaction between COVID-19 and TB may be challenging. It may change the future of TB care. As risk factors, TB and MDR-TB may lead to poor prognosis
and worse COVID-19 outcomes, particularly in the elderly. A great deal of effort should be put into screening and treating active TB infection in hospitalized COVID-19 patients.
ACKNOWLEDGEMENTS
None.
DECLARATIONS
Not applicable.
Ethics approvals and consent to participate
Not applicable.
Conflicts of interest
The authors declare that there is no conflict of interest.
Full-Text: (603 Views)
ABSTRACT
Tuberculosis (TB) has infected billions of people worldwide. The clinical appearance of TB is close to that of Coronavirus disease 2019 (COVID-19). Active pulmonary TB infection can lead to severe pulmonary distress syndrome. Recent studies have revealed the clinical significance of TB surveillance in COVID-19 patients. This mini-review compiled data from published literature and addressed the significance of interplay between TB and SARS-CoV-2 co-infection. Bilateral cross-relationship between these two major public health issues can be classified into two main categories. On the one hand, active TB and multidrug-resistance infections lead to poor prognosis, particularly in elderly patients with COVID-19. On the other hand, the SARS-CoV-2 pandemic caused major harm to the global TB services and surveillance.
Tuberculosis (TB) has infected billions of people worldwide. The clinical appearance of TB is close to that of Coronavirus disease 2019 (COVID-19). Active pulmonary TB infection can lead to severe pulmonary distress syndrome. Recent studies have revealed the clinical significance of TB surveillance in COVID-19 patients. This mini-review compiled data from published literature and addressed the significance of interplay between TB and SARS-CoV-2 co-infection. Bilateral cross-relationship between these two major public health issues can be classified into two main categories. On the one hand, active TB and multidrug-resistance infections lead to poor prognosis, particularly in elderly patients with COVID-19. On the other hand, the SARS-CoV-2 pandemic caused major harm to the global TB services and surveillance.
Keywords: SARS-CoV-2; Tuberculosis; Prognosis
INTRODUCTION
A newly emerged Coronavirus named severe acute respiratory syndrome virus 2 (SARS-CoV-2), an etiologic agent of Coronavirus disease 2019 (COVID-19), is highly infectious and causes an ongoing pandemic. Unlike other human coronaviruses, SARS-CoV-2 is genetically divergent and not geographically limited. COVID-19 patients may be asymptomatic or have mild to severe clinical symptoms. Since the first detection of SARS-CoV-2 in China and the progressive reproductive rate of the virus, millions of people have been infected worldwide. The mortality rate of SARS-CoV-2 (3.4%) is lower than that of SARS and Middle-East respiratory syndrome (MERS) (1). SARS-CoV-2 infection begins in the upper respiratory tract by binding of viral spike glycoprotein to its human receptor angiotensin-converting enzyme 2 (ACE2) (2,3), which is expressed in almost all body tissues, including oral and nasal mucosa, nasopharynx, lung, stomach, small intestine, colon, skin, lymph nodes, thymus, bone marrow, spleen, liver, kidney and brain (4). However, the respiratory route of transmission specifies which organs affect the most and, in this case, the lung is the main organ affected by the SARS-CoV-2 infection. The site of infection is also associated with clinical symptoms.
After 3 to 14 days of incubation, symptomatic cases of COVID-19 may suffer from a variety of clinical symptoms, including fever, cough, myalgia or fatigue, and less frequently headache, hemoptysis, and diarrhea. Severe cases may present with interstitial inflammation, lung lesions, diffuse alveolar damage, cellular fibromyxoid exudates, hyaline membrane formation, and pneumocytes desquamation, indicating acute respiratory distress syndrome (5,6), which may lead to death. Several factors can influence the outcome of COVID-19.
In a cohort study during the pandemic in Scotland, it was noted that the mortality rate
in aged people with diabetes type 1 was 2.39-fold higher than that in the general population (7). In addition, some risk factors, including male sex, hypertension, obesity, smoking, cardiovascular disorders, chronic respiratory disease, cancer (8,9), and co-infection with other human pathogens, should be considered risk factors for increased pathogenicity SARS-CoV-2. Controlling human pathogens in the general population and protecting those with underlying conditions may improve the prognosis of the disease. Acute pulmonary infections such as tuberculosis (TB) also affects lung function in COVID-19 patients (10). In this mini-review, we address the interplay between TB and SARS-CoV-2 co-infection and their bilateral impacts on the disease outcome.
TB and COVID-19: challenges and considerations
Co-infection or superinfection of respiratory viral infections with bacterial pathogens can result in severe symptoms that worsen disease outcomes. SARS-CoV-2 infection can interfere with intestinal homeostasis, favoring bacterial respiratory infection. The important role of the intestinal–lung axis in controlling bacterial pneumonia is well known (11). Bengoechea et al. explained that bacterial co-infection with SARS-CoV-2 may lead to severe inflammatory conditions and tissue damage (11). Therefore, it is important to know what type of bacterial co-infection can occur and to what degree it can affect COVID-19 patients. Furthermore, the elevated occurrence of TB in some underdeveloped and developing countries, along with high population density with poor hygiene, especially in large cities, comprise a scenario that complicates COVID-19 to be foreseen (12,13).
SARS-CoV-2 pneumonia is fatal in susceptible patients. There are, however, bacteria that cause the same disease with
similar symptoms, which must not be ignored. Mycobacterium species is a causative agent of pulmonary TB that has latently infected a fourth of the global population. Here, our current knowledge of the risk of TB to lower the prognosis of COVI-19 patients and vice versa will be argued. One of the early studies was a cohort of 36 COVID-19 patients in China (14). After one month of follow-up, 9/36 patients had severe pneumonia, and 7 (77.8%) were positive for either active or inactive TB. The study proposed TB infection as a risk factor for SARS-CoV-2 infection accompanied by an accelerated development and progression of COVID-19 symptoms. The risk of TB in COVID-19 patients could be even higher than in patients with diabetes, resulting in a poor prognosis. In this regard, Yao et al. reported three cases of COVID-19, of which one patient with no underlying conditions had died of severe pneumonia and active pulmonary TB (15). Singh et al. reported a 76-year-old Indian woman with long-term febrile illness, cough, and shortness of breath (16). Molecular and clinical examinations have demonstrated Co-infection of SARS-CoV-2 with active TB. Therefore, screening and therapeutic management of TB infection in COVID-19 cases are crucial for a better prognosis. In addition to pulmonary TB infection, which needs to be differentiated from SARS-CoV-2 in COVID-19 patients, neurological damage may occur. Ata et al. described a 28-year-old young Indian man with headache, vomiting, and glioma-like brain mass symptoms (17). Anti-TB therapies and follow-up indicated an improved prognosis of TB symptoms and a resolution of COVID-19 symptoms. According to the mentioned study, chest X-ray manifestations of COVID-19 and TB are very much alike, highlighting the necessity of differentiation and screening of patients with TB in COVID-19. In another cohort study of 49 COVID-19 patients from eight countries, a high mortality rate (12.3%) in elderly patients with active TB was reported (18). The findings of this study also indicated that
individuals with active TB were more vulnerable to SARS-CoV-2 infection.
In this regard, another cohort in several European countries demonstrated a higher risk of COVID-19 mortality (11.6%) in elderly patients co-infected with multidrug-resistant (MDR) TB (19). Therefore, an MDR-TB infection should be considered a significant risk factor for the poor prognosis of COVID-19 patients. Tham et al. documented co-infection of COVID-19 with latent TB in four men aged 22 to 40 years (20). The positivity of TB has not been observed in any of the molecular-based methods, and other tests such as interferon-gamma release assay (IGRA) can be used to detect latent TB (20). It is assumed that long-lasting immunity to latent TB confers protection against COVID-19 or decreases the severity of the disease. In this regard, Takahashi assessed the impact of the innate immunity-induced BCG vaccine on the SARS-CoV-2 mortality rate (21). Data revealed a negative association between the prevalence of latent TB infection and the COVID-19 mortality rate. Non-specific innate immunity to latent TB infection can therefore mitigate SARS-CoV-2 infection. Furthermore, early infection of SARS-CoV-2 worsens TB scenarios and global TB services, including decreased screening and surveillance rates, unsuccessful completion of care, and increased TB mortality (22–26). This will considerably burden healthcare workers and clinicians to cope with SARS-CoV-2 and other infectious pathogens.
Comorbidity of COVID-19 and TB
In this study, we have reviewed the literature on the comorbidity of SARS-CoV-2 with TB. Most of the studies concentrate on evaluating the risk of pulmonary TB infection following severe COVID-19. Airborne pathogens such as Mycobacterium tuberculosis may favor infection with SARS-CoV-2 or contribute to the development and progression of symptoms of COVID-19. Co-infection of COVID-19 with TB can lead to hospitalization and poor prognosis (27). Mycobacterium species share risk factors for SARS-CoV-2, including poverty, overcrowding, pollution, and diabetes (28). As a consequence, underdeveloped and developing countries may be encumbered with TB and SARS-CoV-2 co-infections.
M. tuberculosis, Mycobacterium bovis, and Mycobacterium africanum can cause pulmonary TB in low-income countries (29). Co-infection of SARS-CoV-2 can also increase the TB mortality rate by up to 20% for five years (30). However, the intervention of recent anti-SARS-CoV-2 vaccines may affect this outcome. According to a relatively similar manifestation of chest radiography, it may be possible to misdiagnose active TB infection with COVID-19. However, the high-resolution imaging of chest X-rays distinguishes several nodular opacities associated with TB from the ground-glass opacities of COVID-19 (17). Symptoms, including fever and cough, are recorded for both diseases. The differential test for the screening of TB infection should also be carried out. TB could interact with SARS-CoV-2 and impact the mental and physical wellbeing of patients (28,31). The inverse situation is more obscure, and strict lock-downs during the COVID-19 pandemic have led to discrepancies in screening endemic infectious agents such as TB.
Crosstalk between TB and SARS-CoV-2 can be described in two categories (Figure 1). First, the surveillance of TB is significantly concealed by COVID-19. Second, an active and MDR-TB infection can lead to poor prognosis and increased mortality rate following COVID-19. This will change the future outlook for TB elimination and put a severe burden on healthcare workers. Thus, this obstacle has to be addressed to allow patients access to regular TB screening and care. In addition, the promotion and accessibility of the recent anti-SARS-CoV-2 vaccine may reduce the risk of TB infection in hospitalized COVID-19 patients.
A newly emerged Coronavirus named severe acute respiratory syndrome virus 2 (SARS-CoV-2), an etiologic agent of Coronavirus disease 2019 (COVID-19), is highly infectious and causes an ongoing pandemic. Unlike other human coronaviruses, SARS-CoV-2 is genetically divergent and not geographically limited. COVID-19 patients may be asymptomatic or have mild to severe clinical symptoms. Since the first detection of SARS-CoV-2 in China and the progressive reproductive rate of the virus, millions of people have been infected worldwide. The mortality rate of SARS-CoV-2 (3.4%) is lower than that of SARS and Middle-East respiratory syndrome (MERS) (1). SARS-CoV-2 infection begins in the upper respiratory tract by binding of viral spike glycoprotein to its human receptor angiotensin-converting enzyme 2 (ACE2) (2,3), which is expressed in almost all body tissues, including oral and nasal mucosa, nasopharynx, lung, stomach, small intestine, colon, skin, lymph nodes, thymus, bone marrow, spleen, liver, kidney and brain (4). However, the respiratory route of transmission specifies which organs affect the most and, in this case, the lung is the main organ affected by the SARS-CoV-2 infection. The site of infection is also associated with clinical symptoms.
After 3 to 14 days of incubation, symptomatic cases of COVID-19 may suffer from a variety of clinical symptoms, including fever, cough, myalgia or fatigue, and less frequently headache, hemoptysis, and diarrhea. Severe cases may present with interstitial inflammation, lung lesions, diffuse alveolar damage, cellular fibromyxoid exudates, hyaline membrane formation, and pneumocytes desquamation, indicating acute respiratory distress syndrome (5,6), which may lead to death. Several factors can influence the outcome of COVID-19.
In a cohort study during the pandemic in Scotland, it was noted that the mortality rate
in aged people with diabetes type 1 was 2.39-fold higher than that in the general population (7). In addition, some risk factors, including male sex, hypertension, obesity, smoking, cardiovascular disorders, chronic respiratory disease, cancer (8,9), and co-infection with other human pathogens, should be considered risk factors for increased pathogenicity SARS-CoV-2. Controlling human pathogens in the general population and protecting those with underlying conditions may improve the prognosis of the disease. Acute pulmonary infections such as tuberculosis (TB) also affects lung function in COVID-19 patients (10). In this mini-review, we address the interplay between TB and SARS-CoV-2 co-infection and their bilateral impacts on the disease outcome.
TB and COVID-19: challenges and considerations
Co-infection or superinfection of respiratory viral infections with bacterial pathogens can result in severe symptoms that worsen disease outcomes. SARS-CoV-2 infection can interfere with intestinal homeostasis, favoring bacterial respiratory infection. The important role of the intestinal–lung axis in controlling bacterial pneumonia is well known (11). Bengoechea et al. explained that bacterial co-infection with SARS-CoV-2 may lead to severe inflammatory conditions and tissue damage (11). Therefore, it is important to know what type of bacterial co-infection can occur and to what degree it can affect COVID-19 patients. Furthermore, the elevated occurrence of TB in some underdeveloped and developing countries, along with high population density with poor hygiene, especially in large cities, comprise a scenario that complicates COVID-19 to be foreseen (12,13).
SARS-CoV-2 pneumonia is fatal in susceptible patients. There are, however, bacteria that cause the same disease with
similar symptoms, which must not be ignored. Mycobacterium species is a causative agent of pulmonary TB that has latently infected a fourth of the global population. Here, our current knowledge of the risk of TB to lower the prognosis of COVI-19 patients and vice versa will be argued. One of the early studies was a cohort of 36 COVID-19 patients in China (14). After one month of follow-up, 9/36 patients had severe pneumonia, and 7 (77.8%) were positive for either active or inactive TB. The study proposed TB infection as a risk factor for SARS-CoV-2 infection accompanied by an accelerated development and progression of COVID-19 symptoms. The risk of TB in COVID-19 patients could be even higher than in patients with diabetes, resulting in a poor prognosis. In this regard, Yao et al. reported three cases of COVID-19, of which one patient with no underlying conditions had died of severe pneumonia and active pulmonary TB (15). Singh et al. reported a 76-year-old Indian woman with long-term febrile illness, cough, and shortness of breath (16). Molecular and clinical examinations have demonstrated Co-infection of SARS-CoV-2 with active TB. Therefore, screening and therapeutic management of TB infection in COVID-19 cases are crucial for a better prognosis. In addition to pulmonary TB infection, which needs to be differentiated from SARS-CoV-2 in COVID-19 patients, neurological damage may occur. Ata et al. described a 28-year-old young Indian man with headache, vomiting, and glioma-like brain mass symptoms (17). Anti-TB therapies and follow-up indicated an improved prognosis of TB symptoms and a resolution of COVID-19 symptoms. According to the mentioned study, chest X-ray manifestations of COVID-19 and TB are very much alike, highlighting the necessity of differentiation and screening of patients with TB in COVID-19. In another cohort study of 49 COVID-19 patients from eight countries, a high mortality rate (12.3%) in elderly patients with active TB was reported (18). The findings of this study also indicated that
individuals with active TB were more vulnerable to SARS-CoV-2 infection.
In this regard, another cohort in several European countries demonstrated a higher risk of COVID-19 mortality (11.6%) in elderly patients co-infected with multidrug-resistant (MDR) TB (19). Therefore, an MDR-TB infection should be considered a significant risk factor for the poor prognosis of COVID-19 patients. Tham et al. documented co-infection of COVID-19 with latent TB in four men aged 22 to 40 years (20). The positivity of TB has not been observed in any of the molecular-based methods, and other tests such as interferon-gamma release assay (IGRA) can be used to detect latent TB (20). It is assumed that long-lasting immunity to latent TB confers protection against COVID-19 or decreases the severity of the disease. In this regard, Takahashi assessed the impact of the innate immunity-induced BCG vaccine on the SARS-CoV-2 mortality rate (21). Data revealed a negative association between the prevalence of latent TB infection and the COVID-19 mortality rate. Non-specific innate immunity to latent TB infection can therefore mitigate SARS-CoV-2 infection. Furthermore, early infection of SARS-CoV-2 worsens TB scenarios and global TB services, including decreased screening and surveillance rates, unsuccessful completion of care, and increased TB mortality (22–26). This will considerably burden healthcare workers and clinicians to cope with SARS-CoV-2 and other infectious pathogens.
Comorbidity of COVID-19 and TB
In this study, we have reviewed the literature on the comorbidity of SARS-CoV-2 with TB. Most of the studies concentrate on evaluating the risk of pulmonary TB infection following severe COVID-19. Airborne pathogens such as Mycobacterium tuberculosis may favor infection with SARS-CoV-2 or contribute to the development and progression of symptoms of COVID-19. Co-infection of COVID-19 with TB can lead to hospitalization and poor prognosis (27). Mycobacterium species share risk factors for SARS-CoV-2, including poverty, overcrowding, pollution, and diabetes (28). As a consequence, underdeveloped and developing countries may be encumbered with TB and SARS-CoV-2 co-infections.
M. tuberculosis, Mycobacterium bovis, and Mycobacterium africanum can cause pulmonary TB in low-income countries (29). Co-infection of SARS-CoV-2 can also increase the TB mortality rate by up to 20% for five years (30). However, the intervention of recent anti-SARS-CoV-2 vaccines may affect this outcome. According to a relatively similar manifestation of chest radiography, it may be possible to misdiagnose active TB infection with COVID-19. However, the high-resolution imaging of chest X-rays distinguishes several nodular opacities associated with TB from the ground-glass opacities of COVID-19 (17). Symptoms, including fever and cough, are recorded for both diseases. The differential test for the screening of TB infection should also be carried out. TB could interact with SARS-CoV-2 and impact the mental and physical wellbeing of patients (28,31). The inverse situation is more obscure, and strict lock-downs during the COVID-19 pandemic have led to discrepancies in screening endemic infectious agents such as TB.
Crosstalk between TB and SARS-CoV-2 can be described in two categories (Figure 1). First, the surveillance of TB is significantly concealed by COVID-19. Second, an active and MDR-TB infection can lead to poor prognosis and increased mortality rate following COVID-19. This will change the future outlook for TB elimination and put a severe burden on healthcare workers. Thus, this obstacle has to be addressed to allow patients access to regular TB screening and care. In addition, the promotion and accessibility of the recent anti-SARS-CoV-2 vaccine may reduce the risk of TB infection in hospitalized COVID-19 patients.


Figure 1. Schematic representation of crosstalk between TB and COVID-19. The future of TB elimination and better COVID-19 prognosis are closely interlinked
We have also suggested the assessment of the efficiency of COVID-19 vaccination in patients co-infected with TB. Until the mass vaccination program, good prognosis practices need to be implemented to minimize transmission and mortality of SARS-CoV-2.
CONCLUSION
Managing interaction between COVID-19 and TB may be challenging. It may change the future of TB care. As risk factors, TB and MDR-TB may lead to poor prognosis
and worse COVID-19 outcomes, particularly in the elderly. A great deal of effort should be put into screening and treating active TB infection in hospitalized COVID-19 patients.
ACKNOWLEDGEMENTS
None.
DECLARATIONS
Not applicable.
Ethics approvals and consent to participate
Not applicable.
Conflicts of interest
The authors declare that there is no conflict of interest.
Article Type: Review |
Subject:
Microbiology
References
1. Roussel Y, Giraud-Gatineau A, Jimeno MT, Rolain JM, Zandotti C, Colson P, et al. SARS-CoV-2: fear versus data. Int J Antimicrob Agents. 2020;55(5). [View at Publisher] [DOI] [PubMed] [Google Scholar]
2. Mohebbi A, Askari FS, Ebrahimi M, Zakeri M, Yasaghi M, Bagheri H, et al. Susceptibility of the Iranian population to severe acute respiratory syndrome coronavirus 2 infection based on variants of angiotensin i converting enzyme 2. Future Virol. 2020;15(8):507-14. [View at Publisher] [DOI] [Google Scholar]
3. Mohebbi A, Askari FS, Sammak AS, Ebrahimi M, Najafimemar Z. Druggability of cavity pockets within SARS-CoV-2 spike glycoprotein and pharmacophore-based drug discovery. Future Virol [Internet]. 2021 Jun 1 [cited 2021 Jun 4];fvl-2020-0394. Available from: https://www.futuremedicine.com/doi/10.2217/fvl-2020-0394 [View at Publisher] [Google Scholar]
4. Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631-7. [View at Publisher] [DOI] [PubMed] [Google Scholar]
5. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420-2. [View at Publisher] [DOI] [PubMed] [Google Scholar]
6. Varghese G, John R, Manesh A, Karthik R, Abraham O. Clinical management of COVID-19. Indian J Med Res. 2020;151(5):401-10. [View at Publisher] [DOI] [PubMed] [Google Scholar]
7. McGurnaghan SJ, Weir A, Bishop J, Kennedy S, Blackbourn LAK, McAllister DA, et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2020; [View at Publisher] [PubMed] [Google Scholar]
8. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 2020;368. [View at Publisher] [DOI] [PubMed] [Google Scholar]
9. Jordan RE, Adab P, Cheng KK. Covid-19: Risk factors for severe disease and death. BMJ. 2020;368. [View at Publisher] [DOI] [PubMed] [Google Scholar]
10. He G, Wu J, Shi J, Dai J, Gamber M, Jiang X, et al. COVID-19 in tuberculosis patients: A report of three cases. J Med Virol. 2020;92(10):1802-6. [View at Publisher] [DOI] [PubMed] [Google Scholar]
11. Bengoechea JA, Bamford CG. SARS ‐CoV‐2, bacterial co‐infections, and AMR : the deadly trio in COVID ‐19? . EMBO Mol Med. 2020;12(7). [View at Publisher] [DOI] [PubMed] [Google Scholar]
12. Maciel ELN, Gonçalves Júnior E, Dalcolmo MMP. Tuberculose e coronavírus: o que sabemos? Epidemiol e Serv saude Rev do Sist Unico Saude do Bras. 2020;29(2):e2020128. [View at Publisher] [DOI] [PubMed] [Google Scholar]
13. Husain AA, Monaghan TM, Kashyap RS. Impact of COVID-19 pandemic on tuberculosis care in India. Clin Microbiol Infect. 2021;27(2):293-4. [View at Publisher] [DOI] [PubMed] [Google Scholar]
14. Chen Y, Wang Y, Fleming J, Yu Y, Gu Y, Liu C, et al. Active or latent tuberculosis increases susceptibility to COVID-19 and disease severity. medRxiv. 2020; [View at Publisher] [DOI] [Google Scholar]
15. Chen ZYJ, Wang Q, Liu W, Nan QZJ, Huang H, Wu Y, et al. Three Patients with COVID-19 and Pulmonary Tuberculosis, Wuhan, China, January-February 2020. Emerg Infect Dis. 2020;26(11). [View at Publisher] [DOI] [PubMed] [Google Scholar]
16. Singh A, Prasad R, Gupta A, Das K, Gupta N. Severe acute respiratory syndrome coronavirus-2 and pulmonary tuberculosis: convergence can be fatal. Monaldi Arch Chest Dis. 2020;9(3):441-50. [View at Publisher] [DOI] [PubMed] [Google Scholar]
17. Ata F, Yousaf Q, Parambil JV, Parengal J, Mohamedali MG, Yousaf Z. A 28-year-old man from India with SARS-CoV-2 and pulmonary tuberculosis co-infection with central nervous system involvement. Am J Case Rep. 2020;21:1-5. [View at Publisher] [DOI] [PubMed] [Google Scholar]
18. Tadolini M, Codecasa LR, García-García JM, Blanc FX, Borisov S, Alffenaar JW, et al. Active tuberculosis, sequelae and COVID-19 co-infection: First cohort of 49 cases. Eur Respir J. 2020;56(1). [View at Publisher] [DOI] [PubMed] [Google Scholar]
19. Motta I, Centis R, D'Ambrosio L, García-García JM, Goletti D, Gualano G, et al. Tuberculosis, COVID-19 and migrants: Preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology. 2020;26(4):233-40. [View at Publisher] [DOI] [PubMed] [Google Scholar]
20. Tham SM, Lim WY, Loh CKL, Premkumar A, Yan B, Kee A, et al. Four Patients with COVID-19 and Tuberculosis, Singapore, April-May 2020. Emerg Infect Dis. 2020;26(11). [View at Publisher] [DOI] [PubMed] [Google Scholar]
21. Takahashi H. Role of latent tuberculosis infections in reduced COVID-19 mortality: Evidence from an instrumental variable method analysis. Med Hypotheses. 2020;144. [View at Publisher] [DOI] [PubMed] [Google Scholar]
22. Lui Q, Lu P, Shen Y, Li C, Wang J, Zhu L, et al. Collateral Impact of the COVID-19 Pandemic on Tuberculosis. Clin Infect Dis [Internet]. 2020;ciaa1289. Available from: [View at Publisher] [DOI] [Google Scholar]
23. Jain VK, Iyengar KP, Samy DA, Vaishya R. Tuberculosis in the era of COVID-19 in India. Diabetes Metab Syndr Clin Res Rev. 2020;14(5):1439-43. [View at Publisher] [DOI] [PubMed] [Google Scholar]
24. Fei H, Yinyin X, Hui C, Ni W, Xin D, Wei C, et al. The impact of the COVID-19 epidemic on tuberculosis control in China. Lancet Reg Heal - West Pacific. 2020;3:100032. [View at Publisher] [DOI] [PubMed] [Google Scholar]
25. Kwak N, Hwang SS, Yima AJ. Effect of COVID-19 on Tuberculosis Notification, South Korea. Emerg Infect Dis. 2020;26(10). [View at Publisher] [DOI] [PubMed] [Google Scholar]
26. Migliori GB, Thong PM, Akkerman O, Alffenaar JW, Álvarez-Navascués F, Assao-Neino MM, et al. Worldwide Effects of Coronavirus Disease Pandemic on Tuberculosis Services, January-April 2020. Emerg Infect Dis. 2020;26(11):2709-12. [View at Publisher] [DOI] [PubMed] [Google Scholar]
27. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. prevalence of comorbidities and its effects in coronavirus disease 2019 patients: A systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-5. [View at Publisher] [DOI] [PubMed] [Google Scholar]
28. Udwadia ZF, Vora A, Tripathi AR, Malu KN, Lange C, Sara Raju R. COVID-19 -Tuberculosis interactions: When dark forces collide. Indian J Tuberc. 2020;67(4):S155-62. [View at Publisher] [DOI] [PubMed] [Google Scholar]
29. Aliyu G, El-Kamary SS, Abimiku A, Ezati N, Mosunmola I, Hungerford L, et al. Mycobacterial Etiology of Pulmonary Tuberculosis and Association with HIV Infection and Multidrug Resistance in Northern Nigeria. Tuberc Res Treat. 2013;2013:1-9. [View at Publisher] [DOI] [PubMed] [Google Scholar]
30. Hogan AB, Jewell BL, Sherrard-Smith E, Vesga JF, Watson OJ, Whittaker C, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Heal. 2020;8(9):e1132-41. [View at Publisher] [PubMed] [Google Scholar]
31. Nindrea RD, Sari NP, Harahap WA, Haryono SJ, Kusnanto H, Dwiprahasto I, et al. Survey data of multidrug-resistant tuberculosis, Tuberculosis patients characteristics and stress resilience during COVID-19 pandemic in West Sumatera Province, Indonesia. Data Br. 2020;32. [View at Publisher] [DOI] [PubMed] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).





