Volume 9, Issue 1 (Journal of Clinical and Basic Research (JCBR) 2025)
jcbr 2025, 9(1): 14-19 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Amedu N O, Ifelola B. Impact of ethinylestradiol and levonorgestrel on memory, neurochemical indicators, and neuron density in the hippocampus of Wistar rats. jcbr 2025; 9 (1) :14-19
URL: http://jcbr.goums.ac.ir/article-1-509-en.html
URL: http://jcbr.goums.ac.ir/article-1-509-en.html
1- Department of Anatomy, Faculty of Basic Medical Sciences, Adeleke University, Ede, Osun State, Nigeria , amedunath11@gmail.com
2- Department of Anatomy, Faculty of Basic Medical Sciences, Adeleke University, Ede, Osun State, Nigeria
2- Department of Anatomy, Faculty of Basic Medical Sciences, Adeleke University, Ede, Osun State, Nigeria
Full-Text [PDF 577 kb]
(236 Downloads)
| Abstract (HTML) (649 Views)
Discussion
Dopamine plays a crucial role in learning and memory processes across multiple timescales (27). It is involved in various aspects of cognition, including reward signaling, working memory, and long-term plasticity (28). Dopamine's function in learning and memory extends to motivation, prediction error, incentive salience, and memory consolidation (29). Fluctuations in dopamine levels are associated with cognitive deficits in various neurological disorders, including Parkinson's disease, Huntington's disease, schizophrenia, and Alzheimer's disease (28). Acetylcholinesterase (AChE) is an enzyme that degrades acetylcholine (ACh), a key neurotransmitter in learning and memory processes. Recent research suggests that ACh's role in memory is complex and bidirectional, with excessive levels potentially impairing cognitive function (30). The septo-hippocampal cholinergic system plays a crucial role in coordinating multiple memory systems and differentially regulates memory phases, with hippocampal ACh facilitating encoding, but potentially hindering consolidation and retrieval (31). In the current study, prolonged administration of ethinylestradiol and levonorgestrel (21 days) significantly elevated dopamine levels and AChE activity compared to controls, suggesting potential effects on neurotransmission and cognitive functions.
The Y-maze is a behavioral test used to assess spatial working and reference memory in rodents (32). It evaluates spontaneous alternation, a measure of spatial memory (33). The result of this study suggests that prolonged administration (21 days) of ethinylestradiol and levonorgestrel significantly enhances cognitive flexibility in rats, as evidenced by the increased alternation percentage in the Y-maze test. In contrast, shorter durations of administration (7 and 14 days) did not yield statistically significant improvements. Ethinylestradiol and levonorgestrel can affect antioxidant enzymes and cognitive function. Studies show that these hormones can downregulate SOD and catalase genes in various organs, potentially increasing oxidative stress (34). Low doses of these hormones can impair learning and memory while decreasing anxiety-like behavior in rats, possibly due to reduced norepinephrine input to the hippocampus (16). In this study, there was progressive elevation of MDA and decrease in SOD, suggesting a dose- and duration-dependent increase in oxidative stress markers. This also indicates potential suppression of antioxidant defenses following prolonged administration.
Interleukin-1β (IL-1β) is a potent inflammatory cytokine produced in the central nervous system during damage, disease, or stress (35,36). In the hippocampus, IL-1β exhibits cell type-specific signaling, activating the p38 MAPK pathway and CREB in neurons, while triggering NF-κB in astrocytes (36). Elevated hippocampal IL-1β levels are associated with impaired long-term potentiation in aged and stressed rats, potentially linking glucocorticoid, membrane, and free radical theories of aging (37). IL-1β mediates memory impairment during delayed-type hypersensitivity responses, as demonstrated in a study using bacillus Calmette-Guérin in rat hippocampi (38). In this study, the levels of IL-1β were not statistically significant, suggesting minimal impact of ethinylestradiol and levonorgestrel on inflammatory cytokine levels.
The hippocampus is crucial for memory and learning. Quantification of hippocampal CA1 pyramidal cells is important in studying neurodegenerative conditions like Alzheimer's disease (39). The findings highlight the differential vulnerability of hippocampal subregions to pathological conditions and underscore the importance of subregion-specific analyses in understanding hippocampal function and pathology. The results of the current study suggest a time-dependent positive effect of ethinylestradiol and levonorgestrel on pyramidal cell numbers in both CA1 and CA3 regions of the hippocampus. The increase in cell numbers in the CA3 region is more pronounced compared to the CA1 region, indicating differential regional responsiveness to treatment. These results align with potential neuroprotective or neurogenic effects of the administered compounds, warranting further investigation to elucidate underlying mechanisms.
Furthermore, this study shows that CA1 and CA3 regions demonstrate resilience to structural disruption following ethinylestradiol and levonorgestrel administration, as evidenced by the preserved neuronal arrangement across groups. The distortion in the DG highlights a region-specific vulnerability, suggesting differential impacts of the treatment on hippocampal subfields. The findings underscore the importance of further histological and molecular investigations to explore the mechanisms underlying the observed disorganization in the DG.
Conclusion
This study shows that prolonged treatment with ethinylestradiol and levonorgestrel (EE/LNG) improves cognitive flexibility in rats, as indicated by higher Y-maze alternation percentages after 21 days. Neurochemical analysis revealed increased dopamine levels, elevated oxidative stress, reduced antioxidant activity (SOD), and higher acetylcholinesterase (AChE) activity, suggesting altered cholinergic function. Histological examination indicated no structural changes in the CA1 and CA3 hippocampal regions but observed alterations in the dentate gyrus (DG), hinting at potential effects on neurogenesis and hippocampal structure. While EE/LNG positively impacts cognitive and neurochemical functions, it also raises oxidative stress and induces DG-specific structural changes, highlighting the need for further investigation into its long-term effects.
Acknowledgement
The authors wish to express their gratitude to Brainwill Laboratory Osogbo for the valuable technical assistance provided during this study.
Funding sources
This research did not receive any funding from funding agencies in the public, commercial, or non-profit sectors.
Ethical statement
This study was approved by the Ethical and Review Committee of Adeleke University, with approval number AUERC/1259.
Conflicts of interest
Authors declares no conflicts of interests.
Author contributions
Conceptualization and study design: Nathaniel Ohiemi Amedu; Data collection: Blessing Ifelola; Writing the original draft: Nathaniel Ohiemi Amedu and Blessing Ifelola; Review & editing: Nathaniel Ohiemi Amedu, and Blessing Ifelola.
Data availability statement
Data are available upon reasonable request.
Full-Text: (224 Views)
Introduction
Dopamine is a neurotransmitter and hormone belonging to the catecholamine family (1). It plays crucial roles in reward-motivated behavior, movement control, cognition, and emotion (1,2). Dopamine is primarily synthesized from DOPA-by-DOPA decarboxylase, with tyrosine hydroxylase as the rate-limiting enzyme (1). Dopamine dysfunction has been implicated in various neurological and psychiatric disorders, including Parkinson's disease, schizophrenia, and addiction (1-3). In addition to its roles in the central nervous system, dopamine also functions in peripheral tissues, such as the kidneys, where it regulates sodium extraction and electrolyte balance (4). Oxidative stress has been linked to dopamine dysfunction, suggesting potential therapeutic interventions using antioxidant compounds (5).
Oxidative stress, a state in which the balance between free radicals and antioxidants is disrupted, is another area impacted by oral contraceptives (OCs) (6,7). Dopamine itself can contribute to oxidative stress through auto-oxidation and enzymatic oxidation, generating reactive oxygen species and toxic quinones (8,9). These processes are linked to mitochondrial dysfunction, inflammation, and impaired protein degradation (10). The interplay between oxidative stress, mitochondrial dysfunction, and inflammation creates a cascade of events leading to dopaminergic cell death.
Interleukin-1 (IL-1) and acetylcholinesterase (AChE) play interconnected roles in neuroinflammation and neurotransmission. IL-1 overexpression, observed in Alzheimer's disease, can enhance AChE activity and mRNA expression through a cascade involving β-amyloid precursor protein and microglial activation (11). Elevated AChE activity is associated with various inflammatory conditions and may serve as a marker of low-grade systemic inflammation (12). Interestingly, AChE inhibitors can reduce IL-1β production in the brain and blood, suggesting a potential anti-inflammatory mechanism for these drugs (13). The relationship between IL-1 and dopamine is evidenced by the finding that early-life exposure to IL-1β can lead to long-lasting reductions in dopamine content in the hypothalamus and sympathetic ganglia of adult mice (14). These findings highlight the complex interplay between IL-1, AChE, and dopamine in neuroinflammatory processes and neurotransmitter regulation.
The hippocampus is a brain region crucial for learning and memory (15). Ethinylestradiol and levonorgestrel are synthetic hormones commonly used in oral contraceptives. Studies have shown that these hormones can affect hippocampal function and cognitive performance. Low doses of ethinylestradiol and levonorgestrel impaired performance on novel object recognition tests and reduced brain-derived neurotrophic factor mRNA in the hippocampus (16). Additionally, these contraceptives were found to decrease spatial learning ability and alter hippocampal CA3 microstructure, potentially reducing neuronal metabolic activity (17). Levonorgestrel alone biased rats toward using place memory, a hippocampus-mediated process, during navigation tasks. However, this effect was not observed when levonorgestrel was combined with ethinylestradiol (18). These findings suggest that ethinylestradiol and levonorgestrel can influence hippocampal function and cognitive processes, potentially impacting learning and memory in users of oral contraceptives.
Despite these findings, the question remains: How does dopamine regulate the oxidative pathway, interleukin-1, and acetylcholinesterase activity in the hippocampus of Wistar rats following the administration of ethinylestradiol and levonorgestrel? Furthermore, how do these interplays impact learning and memory functions? The aim of this study was to examine how ethinylestradiol and levonorgestrel (EE/LNG) administration affects cognitive flexibility, neurochemical changes, and structural alterations in the rat hippocampus.
Methods
Animals
Forty adolescent female Wistar rats, each weighing an average of 144 g, were sourced from Temilola Animal Husbandry in Osogbo, Osun State, Nigeria. The rats underwent a 14-day acclimatization period in the animal housing facility of the Faculty of Basic Medical Sciences, Adeleke University, Ede. They were kept under a 12-hour light/dark cycle at an ambient temperature of approximately 29 °C and had unrestricted access to food and water. Care for the animals adhered to established guidelines for the use of laboratory animals in biomedical research (19). The experimental protocols were approved by the Adeleke University Ethical and Review Committee with the approval number AUERC/1259.
Experimental design
Ethinylestradiol (0.03 mg) and levonorgestrel (0.15 mg) tablets (Levofem, produced by PT. Harsen Laboratories, Indonesia, and sourced from DKT Nigeria) were dissolved in 0.3 ml of distilled water for administration to rats. Forty rats were randomly divided into four groups of ten. Group A, the control group, received only distilled water for 21 days. Groups B, C, and D were treated with combined oral contraceptives (COC) containing ethinylestradiol and levonorgestrel at doses of 0.03 mg/kg and 0.15 mg/kg body weight, respectively, for 7, 14, and 21 days. The dosage was determined based on a preliminary pilot study conducted by the researchers.
Y-maze test
This test followed a modified method previously described by Amedu and Obu (15). In summary, rats were placed in a Y-maze, which consisted of three opaque arms of equal length connected at 120-degree angles. Each rat was allowed to explore the maze for seven minutes, during which their patterns of alternation were recorded. An alternation was defined as sequential entries into all three arms without revisiting the same arm consecutively. The percentage of alternation for each rat was calculated as the ratio of correct alternations to total alternations, multiplied by 100.
Sample collection and tests
Brain extraction
Twenty-four hours after the behavioral tests, the rats designated for histopathological analysis (n=3) were anesthetized via intraperitoneal injection of ketamine hydrochloride (50 mg/ml, Bharat Parenterals Ltd., India). Perfusion fixation with neutral buffered formalin was then performed, following the protocol described by Gage et al. (20). Subsequently, the rats were decapitated, and the hippocampi from both hemispheres were excised and post-fixed in neutral buffered formalin for 18 hours before tissue processing and staining.
Tissue staining
Tissue samples were processed and embedded in paraffin blocks. These blocks were then sectioned and stained with Hematoxylin and Eosin (H&E) to prepare routine histoarchitectural slides of the hippocampus, following the methodology detailed by Suvarna et al. (21). The stained sections were analyzed using a light microscope (Olympus Model: XSZ-107BN, New Jersey, USA; and Amscope, MD500, CA, USA).
Neurochemical assays
For the neurochemical assays, rats (n = 7) were euthanized via intraperitoneal injection of ketamine hydrochloride, followed by decapitation. The hippocampi from both hemispheres were excised and homogenized in phosphate-buffered saline (PBS) using a Teflon Potter-Elvehjem homogenizer. The homogenates were then centrifuged at 12,000 g for 10 minutes. The resulting supernatants were decanted into test tubes and used to measure malondialdehyde (MDA), superoxide dismutase (SOD), interleukin-1 beta (IL-1β), acetylcholinesterase (AchE), and dopamine levels. MDA, a marker of oxidative stress, was assessed using the thiobarbituric acid reactive substances (TBARS) assay (22,23). The absorbance of the resulting complex was measured at 532 nm. SOD activity, indicative of antioxidant defense, was measured using colorimetric assays (22), with absorbance recorded at 450 nm. IL-1β, a pro-inflammatory cytokine, was quantified using enzyme-linked immunosorbent assay (ELISA) (22), with absorbance measured at 450 nm. Dopamine levels were determined using the technique described by Wang et al. (24). The activity of acetylcholinesterase was measured using the colorimetric method outlined by Ellman et al. (25), with absorbance recorded at 412 nm.
Stereology
Morphometric analyses were conducted on H&E-stained sections using ImageJ software. Five sections from each tissue block were examined across six different visual fields, with magnifications ranging from 25 to 180 µm. For the H&E-stained sections, the number of pyramidal cells (PCs) in the cornu ammonis (CA-1 and -3) region was counted for each group (15). The total numbers of cells in defined regions of the hippocampal formation were determined using the Cavalieri principle, which was used to determine the volumes of the various subdivisions of the rat hippocampus, and the ‘physical disector’ method, which was used to estimate the numerical density of neurons within each subdivision (26). The formula is V = PutN/n. Each point, representing a specific area u, was assessed by counting P, the total number of test points within each distinct subdivision of the sampled sections from a given hippocampus. The volume V of the subdivision was determined using the following relationship. Here, N denotes the total number of serial sections through the hippocampus, n is the number of sampled sections used for point counting, and t represents the average thickness of the serial sections (26).
Statistical analyses
Data were analyzed using GraphPad Prism software (Version 9). Statistical comparisons were carried out through one-way Analysis of Variance (ANOVA) followed by Tukey's multiple comparison test. The results are presented as mean ± SD, with statistical significance defined as P <0.05.
Results
Effects of ethinylestradiol and levonorgestrel administration on cognitive flexibility in the Y-Maze test
Table 1 presents the mean percentage of alternations in the Y-maze test across four experimental groups: the control group, the EE/LNG 7 group (7-day administration of ethinylestradiol and levonorgestrel), the EE/LNG 14 group (14-day administration), and the EE/LNG 21 group (21-day administration). The control group demonstrated an average alternation percentage of 71% ± 10.1, representing the baseline for cognitive flexibility in the Y-maze test. The EE/LNG 7 group exhibited a slightly reduced alternation percentage of 69% ± 17.6, suggesting no significant improvement or decline compared to the control group. The EE/LNG 14 group showed a marginal increase in alternation percentage (75% ± 16.3) compared to the control group, though this difference was not statistically significant. The EE/LNG 21 group achieved the highest alternation percentage (82% ± 14.4), with statistically significant differences (P <0.05) compared to both control and EE/LNG 7 groups. These results suggest that prolonged administration (21 days) of ethinylestradiol and levonorgestrel significantly enhances cognitive flexibility in rats, as evidenced by the increased alternation percentage in the Y-maze test. In contrast, shorter durations of administration (7 and 14 days) did not yield statistically significant improvements.
Neurochemical marker alterations in rats following varying durations of ethinylestradiol and levonorgestrel administration
Table 2 summarizes the mean concentrations of various neurochemical markers in rats across four experimental groups: the control group, the EE/LNG 7 group (7-day administration of ethinylestradiol and levonorgestrel), the EE/LNG 14 group (14-day administration), and the EE/LNG 21 group (21-day administration). Dopamine levels in the control group exhibited a baseline concentration of 397 ± 50 pg/mL. These levels increased progressively across the experimental groups: 423 ± 35 pg/mL in EE/LNG 7, 457 ± 67 pg/mL in EE/LNG 14, and 495 ± 90 pg/mL in EE/LNG 21. A statistically significant increase (P <0.05) was observed in the EE/LNG 21 group compared to the control group, suggesting potential modulatory effects of prolonged administration on dopamine levels.
Malondialdehyde (MDA) levels, an indicator of oxidative stress, were 0.46 ± 0.01 µM/mg in the Control group. These levels increased modestly across the EE/LNG groups, with the highest concentration of 0.6 ± 0.08 µM/mg recorded in EE/LNG 21. This progressive elevation suggests a dose- and duration-dependent increase in oxidative stress markers. Superoxide dismutase (SOD) activity was highest in the control group (1.9 ± 0.08 µM/mg) and decreased progressively with EE/LNG administration. The lowest activity (1.1 ± 0.01 µM/mg) was recorded in EE/LNG 21, indicating potential suppression of antioxidant defenses following prolonged administration.
Interleukin-1β (IL-1β) concentrations were 133 ± 25 pg/mL in the control group, with minor variations across the EE/LNG groups. The highest value (140 ± 23 pg/mL) was observed in EE/LNG 21. These changes were not statistically significant, suggesting minimal impact on inflammatory cytokine levels. Acetylcholinesterase (AChE) activity in the control group was 68 ± 14 nmol/L at baseline. Activity increased progressively with the duration of administration, peaking at 120 ± 24 nmol/L in EE/LNG 21. Significant differences were observed in the EE/LNG 21 group compared to both the control group (P <0.05) and the EE/LNG 7 group (P <0.05), indicating enhanced cholinergic activity with prolonged treatment.
Effects of ethinylestradiol and levonorgestrel on pyramidal cell counts in the hippocampal CA1 and CA3 regions
Table 3 presents the mean number of pyramidal cells in two hippocampal regions (CA1 and CA3) across four experimental groups: the control group, the EE/LNG 7 group (7-day administration of Ethinylestradiol and Levonorgestrel), the EE/LNG 14 group (14-day administration), and the EE/LNG 21 group (21-day administration). In the CA1 region, the Control group exhibited a baseline count of 178.067 ± 9.539 pyramidal cells. The EE/LNG 7 group demonstrated a slight increase in cell count (178.667 ± 8.327) compared to the Control group. A more pronounced increase was observed in the EE/LNG 14 group (181.769 ± 9.900), while the EE/LNG 21 group showed the highest cell count (183.076 ± 7.900). This progressive increase suggests that extended administration of ethinylestradiol and levonorgestrel may promote cellular maintenance or growth in the CA1 region. In the CA3 region, the control group exhibited a baseline count of 254.667 ± 40.673 pyramidal cells. The EE/LNG 7 group showed a significant increase in cell count (278.762 ± 8.734), indicating an early impact of ethinylestradiol and levonorgestrel on this region. Further increases were noted in the EE/LNG 14 group (280.697 ± 9.120) and the EE/LNG 21 group (284.234 ± 9.205), highlighting a sustained increase in pyramidal cell counts with prolonged administration.
Histopathology observation of hippocampal regions following ethinylestradiol and levonorgestrel administration
Figure 1 illustrates representative photomicrographs of H&E-stained sections of the hippocampus, specifically highlighting the cornu ammonis (CA1 and CA3) and the dentate gyrus (DG) regions, across all experimental groups and the control group. The images were captured at 40x magnification to visualize cellular organization and structural integrity. In the CA1 and CA3 regions of all experimental groups (EE/LNG 7, EE/LNG 14, and EE/LNG 21), the pyramidal neurons are arranged in an orderly and well-aligned manner, comparable to those in the control group. This observation suggests that the administration of ethinylestradiol and levonorgestrel does not adversely affect the structural organization or cellular alignment in these regions of the hippocampus. In contrast, the DG region in the experimental groups appears notably distorted and disorganized compared to the control group. This distortion may reflect treatment-induced effects, potentially linked to changes in neurogenesis, cell migration, or structural integrity associated with the administration of ethinylestradiol and levonorgestrel over varying durations.
Dopamine is a neurotransmitter and hormone belonging to the catecholamine family (1). It plays crucial roles in reward-motivated behavior, movement control, cognition, and emotion (1,2). Dopamine is primarily synthesized from DOPA-by-DOPA decarboxylase, with tyrosine hydroxylase as the rate-limiting enzyme (1). Dopamine dysfunction has been implicated in various neurological and psychiatric disorders, including Parkinson's disease, schizophrenia, and addiction (1-3). In addition to its roles in the central nervous system, dopamine also functions in peripheral tissues, such as the kidneys, where it regulates sodium extraction and electrolyte balance (4). Oxidative stress has been linked to dopamine dysfunction, suggesting potential therapeutic interventions using antioxidant compounds (5).
Oxidative stress, a state in which the balance between free radicals and antioxidants is disrupted, is another area impacted by oral contraceptives (OCs) (6,7). Dopamine itself can contribute to oxidative stress through auto-oxidation and enzymatic oxidation, generating reactive oxygen species and toxic quinones (8,9). These processes are linked to mitochondrial dysfunction, inflammation, and impaired protein degradation (10). The interplay between oxidative stress, mitochondrial dysfunction, and inflammation creates a cascade of events leading to dopaminergic cell death.
Interleukin-1 (IL-1) and acetylcholinesterase (AChE) play interconnected roles in neuroinflammation and neurotransmission. IL-1 overexpression, observed in Alzheimer's disease, can enhance AChE activity and mRNA expression through a cascade involving β-amyloid precursor protein and microglial activation (11). Elevated AChE activity is associated with various inflammatory conditions and may serve as a marker of low-grade systemic inflammation (12). Interestingly, AChE inhibitors can reduce IL-1β production in the brain and blood, suggesting a potential anti-inflammatory mechanism for these drugs (13). The relationship between IL-1 and dopamine is evidenced by the finding that early-life exposure to IL-1β can lead to long-lasting reductions in dopamine content in the hypothalamus and sympathetic ganglia of adult mice (14). These findings highlight the complex interplay between IL-1, AChE, and dopamine in neuroinflammatory processes and neurotransmitter regulation.
The hippocampus is a brain region crucial for learning and memory (15). Ethinylestradiol and levonorgestrel are synthetic hormones commonly used in oral contraceptives. Studies have shown that these hormones can affect hippocampal function and cognitive performance. Low doses of ethinylestradiol and levonorgestrel impaired performance on novel object recognition tests and reduced brain-derived neurotrophic factor mRNA in the hippocampus (16). Additionally, these contraceptives were found to decrease spatial learning ability and alter hippocampal CA3 microstructure, potentially reducing neuronal metabolic activity (17). Levonorgestrel alone biased rats toward using place memory, a hippocampus-mediated process, during navigation tasks. However, this effect was not observed when levonorgestrel was combined with ethinylestradiol (18). These findings suggest that ethinylestradiol and levonorgestrel can influence hippocampal function and cognitive processes, potentially impacting learning and memory in users of oral contraceptives.
Despite these findings, the question remains: How does dopamine regulate the oxidative pathway, interleukin-1, and acetylcholinesterase activity in the hippocampus of Wistar rats following the administration of ethinylestradiol and levonorgestrel? Furthermore, how do these interplays impact learning and memory functions? The aim of this study was to examine how ethinylestradiol and levonorgestrel (EE/LNG) administration affects cognitive flexibility, neurochemical changes, and structural alterations in the rat hippocampus.
Methods
Animals
Forty adolescent female Wistar rats, each weighing an average of 144 g, were sourced from Temilola Animal Husbandry in Osogbo, Osun State, Nigeria. The rats underwent a 14-day acclimatization period in the animal housing facility of the Faculty of Basic Medical Sciences, Adeleke University, Ede. They were kept under a 12-hour light/dark cycle at an ambient temperature of approximately 29 °C and had unrestricted access to food and water. Care for the animals adhered to established guidelines for the use of laboratory animals in biomedical research (19). The experimental protocols were approved by the Adeleke University Ethical and Review Committee with the approval number AUERC/1259.
Experimental design
Ethinylestradiol (0.03 mg) and levonorgestrel (0.15 mg) tablets (Levofem, produced by PT. Harsen Laboratories, Indonesia, and sourced from DKT Nigeria) were dissolved in 0.3 ml of distilled water for administration to rats. Forty rats were randomly divided into four groups of ten. Group A, the control group, received only distilled water for 21 days. Groups B, C, and D were treated with combined oral contraceptives (COC) containing ethinylestradiol and levonorgestrel at doses of 0.03 mg/kg and 0.15 mg/kg body weight, respectively, for 7, 14, and 21 days. The dosage was determined based on a preliminary pilot study conducted by the researchers.
Y-maze test
This test followed a modified method previously described by Amedu and Obu (15). In summary, rats were placed in a Y-maze, which consisted of three opaque arms of equal length connected at 120-degree angles. Each rat was allowed to explore the maze for seven minutes, during which their patterns of alternation were recorded. An alternation was defined as sequential entries into all three arms without revisiting the same arm consecutively. The percentage of alternation for each rat was calculated as the ratio of correct alternations to total alternations, multiplied by 100.
Sample collection and tests
Brain extraction
Twenty-four hours after the behavioral tests, the rats designated for histopathological analysis (n=3) were anesthetized via intraperitoneal injection of ketamine hydrochloride (50 mg/ml, Bharat Parenterals Ltd., India). Perfusion fixation with neutral buffered formalin was then performed, following the protocol described by Gage et al. (20). Subsequently, the rats were decapitated, and the hippocampi from both hemispheres were excised and post-fixed in neutral buffered formalin for 18 hours before tissue processing and staining.
Tissue staining
Tissue samples were processed and embedded in paraffin blocks. These blocks were then sectioned and stained with Hematoxylin and Eosin (H&E) to prepare routine histoarchitectural slides of the hippocampus, following the methodology detailed by Suvarna et al. (21). The stained sections were analyzed using a light microscope (Olympus Model: XSZ-107BN, New Jersey, USA; and Amscope, MD500, CA, USA).
Neurochemical assays
For the neurochemical assays, rats (n = 7) were euthanized via intraperitoneal injection of ketamine hydrochloride, followed by decapitation. The hippocampi from both hemispheres were excised and homogenized in phosphate-buffered saline (PBS) using a Teflon Potter-Elvehjem homogenizer. The homogenates were then centrifuged at 12,000 g for 10 minutes. The resulting supernatants were decanted into test tubes and used to measure malondialdehyde (MDA), superoxide dismutase (SOD), interleukin-1 beta (IL-1β), acetylcholinesterase (AchE), and dopamine levels. MDA, a marker of oxidative stress, was assessed using the thiobarbituric acid reactive substances (TBARS) assay (22,23). The absorbance of the resulting complex was measured at 532 nm. SOD activity, indicative of antioxidant defense, was measured using colorimetric assays (22), with absorbance recorded at 450 nm. IL-1β, a pro-inflammatory cytokine, was quantified using enzyme-linked immunosorbent assay (ELISA) (22), with absorbance measured at 450 nm. Dopamine levels were determined using the technique described by Wang et al. (24). The activity of acetylcholinesterase was measured using the colorimetric method outlined by Ellman et al. (25), with absorbance recorded at 412 nm.
Stereology
Morphometric analyses were conducted on H&E-stained sections using ImageJ software. Five sections from each tissue block were examined across six different visual fields, with magnifications ranging from 25 to 180 µm. For the H&E-stained sections, the number of pyramidal cells (PCs) in the cornu ammonis (CA-1 and -3) region was counted for each group (15). The total numbers of cells in defined regions of the hippocampal formation were determined using the Cavalieri principle, which was used to determine the volumes of the various subdivisions of the rat hippocampus, and the ‘physical disector’ method, which was used to estimate the numerical density of neurons within each subdivision (26). The formula is V = PutN/n. Each point, representing a specific area u, was assessed by counting P, the total number of test points within each distinct subdivision of the sampled sections from a given hippocampus. The volume V of the subdivision was determined using the following relationship. Here, N denotes the total number of serial sections through the hippocampus, n is the number of sampled sections used for point counting, and t represents the average thickness of the serial sections (26).
Statistical analyses
Data were analyzed using GraphPad Prism software (Version 9). Statistical comparisons were carried out through one-way Analysis of Variance (ANOVA) followed by Tukey's multiple comparison test. The results are presented as mean ± SD, with statistical significance defined as P <0.05.
Results
Effects of ethinylestradiol and levonorgestrel administration on cognitive flexibility in the Y-Maze test
Table 1 presents the mean percentage of alternations in the Y-maze test across four experimental groups: the control group, the EE/LNG 7 group (7-day administration of ethinylestradiol and levonorgestrel), the EE/LNG 14 group (14-day administration), and the EE/LNG 21 group (21-day administration). The control group demonstrated an average alternation percentage of 71% ± 10.1, representing the baseline for cognitive flexibility in the Y-maze test. The EE/LNG 7 group exhibited a slightly reduced alternation percentage of 69% ± 17.6, suggesting no significant improvement or decline compared to the control group. The EE/LNG 14 group showed a marginal increase in alternation percentage (75% ± 16.3) compared to the control group, though this difference was not statistically significant. The EE/LNG 21 group achieved the highest alternation percentage (82% ± 14.4), with statistically significant differences (P <0.05) compared to both control and EE/LNG 7 groups. These results suggest that prolonged administration (21 days) of ethinylestradiol and levonorgestrel significantly enhances cognitive flexibility in rats, as evidenced by the increased alternation percentage in the Y-maze test. In contrast, shorter durations of administration (7 and 14 days) did not yield statistically significant improvements.
Neurochemical marker alterations in rats following varying durations of ethinylestradiol and levonorgestrel administration
Table 2 summarizes the mean concentrations of various neurochemical markers in rats across four experimental groups: the control group, the EE/LNG 7 group (7-day administration of ethinylestradiol and levonorgestrel), the EE/LNG 14 group (14-day administration), and the EE/LNG 21 group (21-day administration). Dopamine levels in the control group exhibited a baseline concentration of 397 ± 50 pg/mL. These levels increased progressively across the experimental groups: 423 ± 35 pg/mL in EE/LNG 7, 457 ± 67 pg/mL in EE/LNG 14, and 495 ± 90 pg/mL in EE/LNG 21. A statistically significant increase (P <0.05) was observed in the EE/LNG 21 group compared to the control group, suggesting potential modulatory effects of prolonged administration on dopamine levels.
Malondialdehyde (MDA) levels, an indicator of oxidative stress, were 0.46 ± 0.01 µM/mg in the Control group. These levels increased modestly across the EE/LNG groups, with the highest concentration of 0.6 ± 0.08 µM/mg recorded in EE/LNG 21. This progressive elevation suggests a dose- and duration-dependent increase in oxidative stress markers. Superoxide dismutase (SOD) activity was highest in the control group (1.9 ± 0.08 µM/mg) and decreased progressively with EE/LNG administration. The lowest activity (1.1 ± 0.01 µM/mg) was recorded in EE/LNG 21, indicating potential suppression of antioxidant defenses following prolonged administration.
Interleukin-1β (IL-1β) concentrations were 133 ± 25 pg/mL in the control group, with minor variations across the EE/LNG groups. The highest value (140 ± 23 pg/mL) was observed in EE/LNG 21. These changes were not statistically significant, suggesting minimal impact on inflammatory cytokine levels. Acetylcholinesterase (AChE) activity in the control group was 68 ± 14 nmol/L at baseline. Activity increased progressively with the duration of administration, peaking at 120 ± 24 nmol/L in EE/LNG 21. Significant differences were observed in the EE/LNG 21 group compared to both the control group (P <0.05) and the EE/LNG 7 group (P <0.05), indicating enhanced cholinergic activity with prolonged treatment.
Effects of ethinylestradiol and levonorgestrel on pyramidal cell counts in the hippocampal CA1 and CA3 regions
Table 3 presents the mean number of pyramidal cells in two hippocampal regions (CA1 and CA3) across four experimental groups: the control group, the EE/LNG 7 group (7-day administration of Ethinylestradiol and Levonorgestrel), the EE/LNG 14 group (14-day administration), and the EE/LNG 21 group (21-day administration). In the CA1 region, the Control group exhibited a baseline count of 178.067 ± 9.539 pyramidal cells. The EE/LNG 7 group demonstrated a slight increase in cell count (178.667 ± 8.327) compared to the Control group. A more pronounced increase was observed in the EE/LNG 14 group (181.769 ± 9.900), while the EE/LNG 21 group showed the highest cell count (183.076 ± 7.900). This progressive increase suggests that extended administration of ethinylestradiol and levonorgestrel may promote cellular maintenance or growth in the CA1 region. In the CA3 region, the control group exhibited a baseline count of 254.667 ± 40.673 pyramidal cells. The EE/LNG 7 group showed a significant increase in cell count (278.762 ± 8.734), indicating an early impact of ethinylestradiol and levonorgestrel on this region. Further increases were noted in the EE/LNG 14 group (280.697 ± 9.120) and the EE/LNG 21 group (284.234 ± 9.205), highlighting a sustained increase in pyramidal cell counts with prolonged administration.
Histopathology observation of hippocampal regions following ethinylestradiol and levonorgestrel administration
Figure 1 illustrates representative photomicrographs of H&E-stained sections of the hippocampus, specifically highlighting the cornu ammonis (CA1 and CA3) and the dentate gyrus (DG) regions, across all experimental groups and the control group. The images were captured at 40x magnification to visualize cellular organization and structural integrity. In the CA1 and CA3 regions of all experimental groups (EE/LNG 7, EE/LNG 14, and EE/LNG 21), the pyramidal neurons are arranged in an orderly and well-aligned manner, comparable to those in the control group. This observation suggests that the administration of ethinylestradiol and levonorgestrel does not adversely affect the structural organization or cellular alignment in these regions of the hippocampus. In contrast, the DG region in the experimental groups appears notably distorted and disorganized compared to the control group. This distortion may reflect treatment-induced effects, potentially linked to changes in neurogenesis, cell migration, or structural integrity associated with the administration of ethinylestradiol and levonorgestrel over varying durations.
Table 1. Percentage of alternations by rats in the Y-maze test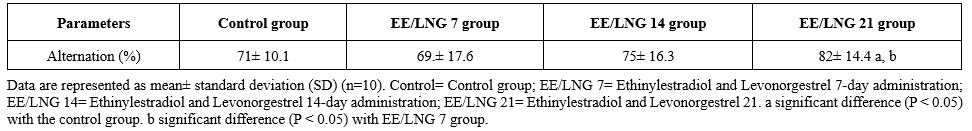 Table 2. The mean concentration of neurochemicals 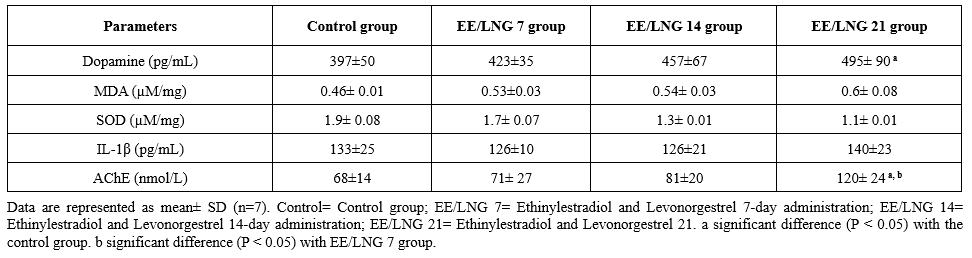 |
|
Table 3. The mean number of pyramidal cells
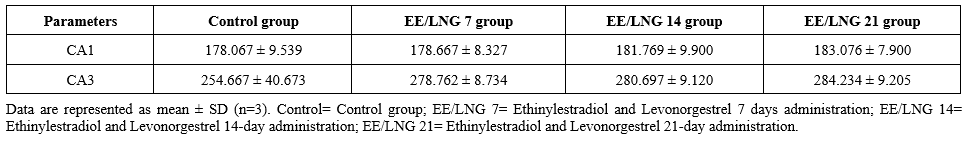 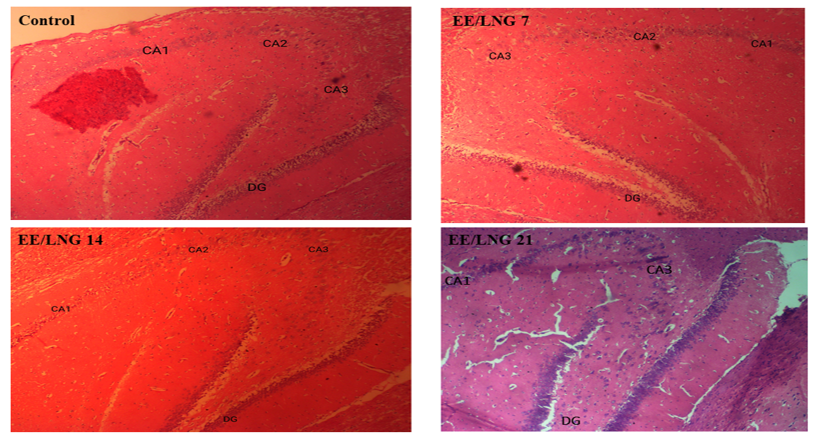 Figure 1. Representative photomicrograph showing H&E-stained sections of the Hippocampus (CA: Cornu Ammonis 1, 3, and DG= Dentate Gyrus). Magnification = X40. At this magnification, CA 1 and 3 in all the experimental groups appear properly arranged similar to the control group. However, the DG in the experimental groups appear more distorted and disorganized than the control group. Control= Control group; EE/LNG 7= Ethinylestradiol and Levonorgestrel 7-day administration; EE/LNG 14= Ethinylestradiol and Levonorgestrel 14-day administration; EE/LNG 21= Ethinylestradiol and Levonorgestrel 21-day administration. |
Discussion
Dopamine plays a crucial role in learning and memory processes across multiple timescales (27). It is involved in various aspects of cognition, including reward signaling, working memory, and long-term plasticity (28). Dopamine's function in learning and memory extends to motivation, prediction error, incentive salience, and memory consolidation (29). Fluctuations in dopamine levels are associated with cognitive deficits in various neurological disorders, including Parkinson's disease, Huntington's disease, schizophrenia, and Alzheimer's disease (28). Acetylcholinesterase (AChE) is an enzyme that degrades acetylcholine (ACh), a key neurotransmitter in learning and memory processes. Recent research suggests that ACh's role in memory is complex and bidirectional, with excessive levels potentially impairing cognitive function (30). The septo-hippocampal cholinergic system plays a crucial role in coordinating multiple memory systems and differentially regulates memory phases, with hippocampal ACh facilitating encoding, but potentially hindering consolidation and retrieval (31). In the current study, prolonged administration of ethinylestradiol and levonorgestrel (21 days) significantly elevated dopamine levels and AChE activity compared to controls, suggesting potential effects on neurotransmission and cognitive functions.
The Y-maze is a behavioral test used to assess spatial working and reference memory in rodents (32). It evaluates spontaneous alternation, a measure of spatial memory (33). The result of this study suggests that prolonged administration (21 days) of ethinylestradiol and levonorgestrel significantly enhances cognitive flexibility in rats, as evidenced by the increased alternation percentage in the Y-maze test. In contrast, shorter durations of administration (7 and 14 days) did not yield statistically significant improvements. Ethinylestradiol and levonorgestrel can affect antioxidant enzymes and cognitive function. Studies show that these hormones can downregulate SOD and catalase genes in various organs, potentially increasing oxidative stress (34). Low doses of these hormones can impair learning and memory while decreasing anxiety-like behavior in rats, possibly due to reduced norepinephrine input to the hippocampus (16). In this study, there was progressive elevation of MDA and decrease in SOD, suggesting a dose- and duration-dependent increase in oxidative stress markers. This also indicates potential suppression of antioxidant defenses following prolonged administration.
Interleukin-1β (IL-1β) is a potent inflammatory cytokine produced in the central nervous system during damage, disease, or stress (35,36). In the hippocampus, IL-1β exhibits cell type-specific signaling, activating the p38 MAPK pathway and CREB in neurons, while triggering NF-κB in astrocytes (36). Elevated hippocampal IL-1β levels are associated with impaired long-term potentiation in aged and stressed rats, potentially linking glucocorticoid, membrane, and free radical theories of aging (37). IL-1β mediates memory impairment during delayed-type hypersensitivity responses, as demonstrated in a study using bacillus Calmette-Guérin in rat hippocampi (38). In this study, the levels of IL-1β were not statistically significant, suggesting minimal impact of ethinylestradiol and levonorgestrel on inflammatory cytokine levels.
The hippocampus is crucial for memory and learning. Quantification of hippocampal CA1 pyramidal cells is important in studying neurodegenerative conditions like Alzheimer's disease (39). The findings highlight the differential vulnerability of hippocampal subregions to pathological conditions and underscore the importance of subregion-specific analyses in understanding hippocampal function and pathology. The results of the current study suggest a time-dependent positive effect of ethinylestradiol and levonorgestrel on pyramidal cell numbers in both CA1 and CA3 regions of the hippocampus. The increase in cell numbers in the CA3 region is more pronounced compared to the CA1 region, indicating differential regional responsiveness to treatment. These results align with potential neuroprotective or neurogenic effects of the administered compounds, warranting further investigation to elucidate underlying mechanisms.
Furthermore, this study shows that CA1 and CA3 regions demonstrate resilience to structural disruption following ethinylestradiol and levonorgestrel administration, as evidenced by the preserved neuronal arrangement across groups. The distortion in the DG highlights a region-specific vulnerability, suggesting differential impacts of the treatment on hippocampal subfields. The findings underscore the importance of further histological and molecular investigations to explore the mechanisms underlying the observed disorganization in the DG.
Conclusion
This study shows that prolonged treatment with ethinylestradiol and levonorgestrel (EE/LNG) improves cognitive flexibility in rats, as indicated by higher Y-maze alternation percentages after 21 days. Neurochemical analysis revealed increased dopamine levels, elevated oxidative stress, reduced antioxidant activity (SOD), and higher acetylcholinesterase (AChE) activity, suggesting altered cholinergic function. Histological examination indicated no structural changes in the CA1 and CA3 hippocampal regions but observed alterations in the dentate gyrus (DG), hinting at potential effects on neurogenesis and hippocampal structure. While EE/LNG positively impacts cognitive and neurochemical functions, it also raises oxidative stress and induces DG-specific structural changes, highlighting the need for further investigation into its long-term effects.
Acknowledgement
The authors wish to express their gratitude to Brainwill Laboratory Osogbo for the valuable technical assistance provided during this study.
Funding sources
This research did not receive any funding from funding agencies in the public, commercial, or non-profit sectors.
Ethical statement
This study was approved by the Ethical and Review Committee of Adeleke University, with approval number AUERC/1259.
Conflicts of interest
Authors declares no conflicts of interests.
Author contributions
Conceptualization and study design: Nathaniel Ohiemi Amedu; Data collection: Blessing Ifelola; Writing the original draft: Nathaniel Ohiemi Amedu and Blessing Ifelola; Review & editing: Nathaniel Ohiemi Amedu, and Blessing Ifelola.
Data availability statement
Data are available upon reasonable request.
Article Type: Research |
Subject:
Neuroscience
References
1. Ando H, Ukena K, Nagata S. Handbook of Hormones: Comparative Endocrinology for Basic and Clinical Research. Academic Press;2021. [View at Publisher] [DOI] [Google Scholar]
2. Speranza L, Di Porzio U, Viggiano D, de Donato A, Volpicelli F. Dopamine: The neuromodulator of long-term synaptic plasticity, reward and movement control. Cells. 2021;10(4):735. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Kienast T, Heinz A. Dopamine and the diseased brain. CNS Neurol Disord Drug Targets. CNS Neurol Disord Drug Targets. 2006;5(1):109-31. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Olivares-Hernández A, Figuero-Pérez L, Cruz-Hernandez JJ, Sarmiento RG, Usategui-Martin R, Miramontes-González JP. Dopamine Receptors and the Kidney: An Overview of Health- and Pharmacological-Targeted Implications. Biomolecules. 2021;11(2):254. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Juárez Olguín H, Calderón Guzmán D, Hernández García E, Barragán Mejía G. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxid Med Cell Longev. 2015;2016:9730467. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Quinn KM, Roberts L, Cox AJ, Borg DN, Pennell EN, McKeating DR, et al. Blood oxidative stress biomarkers in women: influence of oral contraception, exercise, and N-acetylcysteine. Eur J Appl Physiol. 2022;122(8):1949-64. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Cauci S, Xodo S, Buligan C, Colaninno C, Barbina M, Barbina G, et al. Oxidative Stress Is Increased in Combined Oral Contraceptives Users and Is Positively Associated with High-Sensitivity C-Reactive Protein. Molecules. 2021;26(4):1070. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Meiser J, Weindl D, Hiller K. Complexity of dopamine metabolism. Cell Commun Signal. 2013;11(1):34. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Miyazaki I, Asanuma M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself. Acta Medica Okayama. 2008;62(3):141-50. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Jenner P, Hunot, Olanow, Beal, Kordower, Tatton, et al. Oxidative stress in Parkinson's disease. Ann Neurol
. 2003;53(S3):S26-36. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Shaftel SS, Griffin WST, Kerry OM. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Das UN. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med Sci Monit. 2007;13(12):RA214-21. [View at Publisher] [PMID] [Google Scholar]
13. Pollak Y, Gilboa A, Ben-Menachem O, Ben-Hur T, Soreq H, Yirmiya R. Acetylcholinesterase inhibitors reduce brain and blood interleukin-1β production. Ann Neurol. 2005;57(5):741-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Kabiersch A, Furukawa H, Rey A Del, Besedovsky HO. Administration of interleukin-1 at birth affects dopaminergic neurons in adult mice. Ann N Y Acad Sci. 1998:840:123-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Amedu NO, Obu MO. Atrazine-induced Hippocampal Degeneration and Behavioral Deficits in Wistar Rats: Mitigative role of avocado oil. Iranian Journal of Toxicology. 2022;16(3):211-20. [View at Publisher] [DOI] [Google Scholar]
16. Simone J, Bogue EA, Bhatti DL, Day LE, Farr NA, Grossman AM, et al. Ethinyl estradiol and levonorgestrel alter cognition and anxiety in rats concurrent with a decrease in tyrosine hydroxylase expression in the locus coeruleus and brain-derived neurotrophic factor expression in the hippocampus. Psychoneuroendocrinology. 2015;62:265-78. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Nwakanma A, Ekanem T, Eluwa M, Elemuo C, Ekong M. Ethinyl estadiol/progestin oral contraceptives depress spatial learning and dysregulate hippocampal CA3 microstructure: Implications for behavioral-cognitive effects of chronic contraceptive use? Acta Medica Bulgarica. 2021;48(2):53-61. [View at Publisher] [DOI] [Google Scholar]
18. Lacasse JM, Boulos V, Fisher C, Hamilton S, Heron M, Mac Cionnaith CE, et al. Combined effects of the contraceptive hormones, ethinyl estradiol and levonorgestrel, on the use of place and response memory in gonadally-intact female rats. Psychoneuroendocrinology. 2023:147:105974. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Jones-Bolin S. Guidelines for the care and use of laboratory animals in biomedical research. Curr Protoc Pharmacol. 2012;2012:Appendix 4:Appendix 4B. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Gage GJ, Kipke DR, Shain W. Whole animal perfusion fixation for rodents. J Vis Exp. 2012;(65):3564. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Suvarna SK, Layton C, Bancroft JD. Bancroft's Theory and Practice of Histological Techniques. 8th ed. UK:Elsevier Health Sci;2018. [View at Publisher] [Google Scholar]
22. Gao H, Tian Y, Wang W, Yao D, Zheng T, Meng Q. Levels of interleukin-6, superoxide dismutase and malondialdehyde in the lung tissue of a rat model of hypoxia-induced acute pulmonary edema. Exp Ther Med. 2016;11(3):993-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Fajrilah BR, Indrayani UD, Djamâ€TMan Q. The Effect of Honey on Plasma Malondialdehyde (MDA) Level onAlloxan-Induced hyperglycemic Rats An Experimental studies in rats Galur Wistar White Males. Sains Medika : Jurnal Kedokteran dan Kesehatan. 2013;5(2). [View at Publisher] [DOI] [Google Scholar]
24. Wang HB, Li Y, Dong GL, Gan T, Liu YM. A convenient and label-free colorimetric assay for dopamine detection based on the inhibition of the Cu(II)-catalyzed oxidation of a 3,3′,5,5′-tetramethylbenzidine-H2O2 system. New Journal of Chemistry. 2017;41(23). [View at Publisher] [DOI] [Google Scholar]
25. Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7(2):88-95. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Miki T, Satriotomo I, Li HP, Matsumoto Y, Gu H, Yokoyama T, et al. Application of the physical disector to the central nervous system: estimation of the total number of neurons in subdivisions of the rat hippocampus. Anat Sci Int. 2005;80(3):153-62. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Baudonnat M, Huber A, David V, Walton ME. Heads for learning, tails for memory: Reward, reinforcement and a role of dopamine in determining behavioral relevance across multiple timescales. Front Neurosci. 2013:7:175. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Kourosh-Arami M, Komaki A, Zarrindast MR. Dopamine as a Potential Target for Learning and Memory: Contributing to Related Neurological Disorders. CNS Neurol Disord Drug Targets. 2023;22(4):558-76. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Abraham AD, Neve KA, Lattal KM. Dopamine and extinction: A convergence of theory with fear and reward circuitry. Neurobiol Learn Mem. 2014:108:65-77. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Huang Q, Liao C, Ge F, Ao J, Liu T. Acetylcholine bidirectionally regulates learning and memory. Journal of Neurorestoratology. 2022;10(2). [View at Publisher] [DOI] [Google Scholar]
31. Micheau J, Marighetto A. Acetylcholine and memory: A long, complex and chaotic but still living relationship. Behav Brain Res. 2011;221(2):424-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Kraeuter AK, Guest PC, Sarnyai Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol Biol. 2019:1916:105-11. [View at Publisher] [DOI] [PMID] [Google Scholar]
33. Golub M. UC Davis - Y-Maze. v1. 2019. [Protocol] [View at Publisher] [DOI]
34. Wusu AD, Bankole HA, Fatai AA, Kanmodi RI, Obasieke PE, Wusu TD. Combined Oral Administration of Ethinylestradiol and Levonorgestrel Alters the Expression of Antioxidant and Apoptotic Markers in Female Rats. 2021;16(1). [View at Publisher] [Google Scholar]
35. Gajtkó A, Bakk E, Hegedűs K, Ducza L, Holló K. IL-1β Induced Cytokine Expression by Spinal Astrocytes Can Play a Role in the Maintenance of Chronic Inflammatory Pain. Front Physiol. 2020:11:543331. [View at Publisher] [DOI] [PMID] [Google Scholar]
36. Srinivasan D, Yen JH, Joseph DJ, Friedman W. Cell type-specific interleukin-1beta signaling in the CNS. J Neurosci. 2004;24(29):6482-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
37. Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1β is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18(8):2974-81. [View at Publisher] [DOI] [PMID] [Google Scholar]
38. Palin K, Bluthé RM, Verrier D, Tridon V, Dantzer R, Lestage J. Interleukin-1β mediates the memory impairment associated with a delayed type hypersensitivity response to bacillus Calmette-Guérin in the rat hippocampus. Brain Behav Immun. 2004;18(3):223-30. [View at Publisher] [DOI] [PMID] [Google Scholar]
39. Azzubaidi MS, Saxena A, Talib NA. Quantifying dorsal Hippocampal CA-1 pyramidal cells in rats: rules to light microscope based estimation. Malaysian Journal of Microscopy. 2013;9(1). [View at Publisher] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).





