Volume 9, Issue 3 (Journal of Clinical and Basic Research (JCBR) 2025)
jcbr 2025, 9(3): 13-16 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sagarika D, Baseer S, Karamchedu S, Hussaini S A B, Fatima R, Laxmi P R et al . Study of germ cell tumors of ovary and associated immunohistochemistry. jcbr 2025; 9 (3) :13-16
URL: http://jcbr.goums.ac.ir/article-1-479-en.html
URL: http://jcbr.goums.ac.ir/article-1-479-en.html
Devunoori Sagarika1 

 , Shafaq Baseer2
, Shafaq Baseer2 

 , Shilpa Karamchedu3
, Shilpa Karamchedu3 

 , Syed Ali Baqher Hussaini *4
, Syed Ali Baqher Hussaini *4 

 , Rasheed Fatima3
, Rasheed Fatima3 

 , Panala Rajya Laxmi3
, Panala Rajya Laxmi3 

 , Sana Sultana3
, Sana Sultana3 




 , Shafaq Baseer2
, Shafaq Baseer2 

 , Shilpa Karamchedu3
, Shilpa Karamchedu3 

 , Syed Ali Baqher Hussaini *4
, Syed Ali Baqher Hussaini *4 

 , Rasheed Fatima3
, Rasheed Fatima3 

 , Panala Rajya Laxmi3
, Panala Rajya Laxmi3 

 , Sana Sultana3
, Sana Sultana3 


1- Department of Pathology, SVS Medical College, Yenugonda, Mahabubnagar, Telangana, India
2- Department of Pathology, Government Medical College, Mahabubnagar, Telangana, India
3- Department of Pathology, SVS Medical College and Hospital, Yenugonda, Mahabubnagar, Telangana, India
4- S.N Diagnostic Centre, Kalaburagi, Karnataka, India ,vali_shaik31@rediffmail.com
2- Department of Pathology, Government Medical College, Mahabubnagar, Telangana, India
3- Department of Pathology, SVS Medical College and Hospital, Yenugonda, Mahabubnagar, Telangana, India
4- S.N Diagnostic Centre, Kalaburagi, Karnataka, India ,
Keywords: Germ cell tumors, Hematoxylin and eosin, Carcinoma, Embryonal, Choriocarcinoma, Dysgerminoma
Full-Text [PDF 832 kb]
(239 Downloads)
| Abstract (HTML) (720 Views)
Discussion
Ovarian tumors occur most commonly in women of reproductive age, with approximately two-thirds of cases diagnosed in the aforementioned group (10). These tumors often remain clinically silent due to their deep anatomical location, with abdominal mass and pain being the most frequent presenting symptoms. In the present study, the patients were within the age range of 11-70 years, with a mean age of 33 years. The youngest patient was an 11-year-old female with a unilateral mature teratoma (9.5 × 5.5 × 4 cm) presenting with abdominal pain, the most common germ cell tumor in adolescents. The oldest patient was a 70-year-old female with unilateral mucinous cystadenocarcinoma (19 × 14 × 12 cm) presenting with ascites. The majority of cases (77.78%) were within the age range of 21–50 years, consistent with the findings of previous studies (11,12).
Premenopausal women showed a higher prevalence of ovarian tumors, whereas postmenopausal women exhibited a relatively higher prevalence of malignant tumors, consistent with the findings reported by Jha et al. and Kayastha et al. (11,12). The tumors were sometimes incidentally detected on ultrasound; however, symptomatic patients were categorized based on the predominant presenting complaint. In the present study, the most frequent symptom was an abdominal mass (56%), followed by abdominal pain (20%) and menstrual irregularities, including postmenopausal bleeding (16%). The aforementioned results align with the findings of prior studies by Rashid et al. and Jamal et al., which reported abdominal pain and mass as the most common presenting complaints (13,14).
Most tumors in the present cohort were unilateral (96%), with only 4% presenting bilaterally; among bilateral tumors, malignancy was more frequent. The obtained result is similar to the findings reported by Jha et al. (11) and Tushar et al. (15), although the prevalence of bilaterality varied among studies. Tumor sizes ranged from 3 to 30 cm, with 88% measuring less than 20 cm. The largest tumor was a 30 × 25 × 15 cm unilateral benign papillary serous cystadenofibroma, comparable to Pilli et al.’s observations.
Regarding architecture, 64%, 28%, and 8% of the tumors were purely cystic, solid, and mixed, respectively. These proportions are consistent with the findings of Kar et al.; however, differences might be explained by a higher number of malignant tumors in the aforementioned study. Solid or complex tumors were associated with higher malignancy risk, as shown by Timmerman et al. (16). Overall, 68% and 32% of the tumors were benign and malignant, respectively, with benign tumors being predominantly cystic and malignant tumors solid or mixed, in line with the findings of Gupta et al. (17), Madan et al. (18), and Choudhary et al. (19).
Germ cell tumors were classified as benign or malignant based on predominant cell type, growth pattern, stromal content, and cellular atypia with invasiveness. Immunohistochemistry revealed HCG-/CA125-/AFP- expression in dysgerminomas and immature teratomas; nevertheless, endodermal sinus tumors showed HCG-/CA125-/AFP+ expression. The frequencies of benign (68%) and malignant (32%) germ cell tumors were comparable to those of previous studies by Mandal et al. (Benign: 63.1%, malignant: 29.6%) and Chavan et al. (Benign: 70.1%, malignant: 29.9%) (20,21).
In the current study, age was a significant factor; accordingly, the tumors in patients under 40 years were mostly benign, whereas the tumors in patients over 40 showed a higher prevalence of malignancy. Malignant tumors in younger adults were primarily germ cell or mixed germ cell tumors.
Study limitations
The current study was limited by a small sample size, which restricted broader analysis.
Conclusion
The increased prevalence of malignancy was observed in patients over 50 years of age, in the postmenopausal group, in tumors with solid or complex morphology, and in bilateral tumors. The aforementioned parameters might serve as useful predictors of malignancy in ovarian tumors. Among ovarian neoplasms, germ cell tumors are relatively uncommon. Benign tumors are primarily mature cystic teratomas; however, malignant germ cell tumors include dysgerminomas. Choriocarcinomas demonstrate strong HCG positivity, whereas endodermal sinus tumors and embryonal carcinomas show strong AFP expression.
Acknowledgement
None.
Funding sources
Not received any funding.
Ethical statement
This study received approval from the Ethics Committee of SVS Medical College, under an approval code (SVS/IEC/2023/Path/03).
Conflicts of interest
The authors declared no conflict of interest.
Author contributions
DS, SB, SK, SAB, PRL, and SS collected the data. RF designed the protocol and conceptualized the study. All authors revised and analyzed the data. All authors worked together in drafting the manuscript. The final version of the draft was reviewed and approved by all authors.
Data availability statement
Data is available upon request from the corresponding author.
Full-Text: (122 Views)
Introduction
Breast cancer (BC) is the most common malignancy in women worldwide and accounts for approximately 1,000,000 mortalities annually (1). In India, ovarian tumors rank as the fifth leading cause of cancer-related mortality among women (2). Breast cancer represents 6% of all cancers in Indian women, with an annual prevalence of 9 per 100,000 individuals (3). Most breast tumors occur in women aged 25-45 years. Established risk factors include positive family history, genetic mutations, and hormonal imbalances (4). The majority of breast carcinomas originate from the epithelial lining of the ducts or lobules; accordingly, carcinomas are classified as ductal or lobular carcinomas. Histologic grading reflects the degree of differentiation, indicating the resemblance of tumor cells to normal breast tissue (5).
Ovarian tumorigenesis is thought to result from repeated endocrine and mechanical trauma during monthly ovulatory cycles. The cumulative effect of ovulatory rupture and repair might contribute to genetic mutations leading to malignancy. This mechanism explains the protective effects of oral contraceptive use, late menarche, early menopause, multiparity, and breastfeeding, all of which reduce the number of ovulatory cycles (6). Ultrasonography and cancer antigen 125 (CA-125) assessments serve as screening tools for early detection; however, Fine Needle Aspiration Cytology (FNAC) provides primary diagnosis and prognostic information with 90-95% accuracy in differentiating benign from malignant lesions (7). According to the World Health Organization (WHO) guidelines, ovarian tumors are histologically classified as benign, borderline, or malignant, with this classification carrying significant therapeutic and prognostic implications. Variations in histopathological patterns are commonly observed in both primary and secondary ovarian tumors (8).
In BC, ongoing studies are utilizing panels of antibodies, such as estrogen receptor (ER), progesterone receptor (PR), human EGF receptor-2 (HER2)/neu, cytokeratin 5/6, epidermal growth factor receptor (EGFR), and Ki-67, to classify tumors into molecular subgroups (9). Similarly, in ovarian germ cell tumors, alpha-fetoprotein (AFP) is associated with yolk sac elements; however, human chorionic gonadotropin (HCG) serves as a marker of trophoblastic differentiation.
The present study aimed to investigate the histomorphological spectrum and histological grading of ovarian germ cell tumors using the modified Bloom-Richardson Grading System. Furthermore, this study sought to molecularly classify these tumors using AFP, CA-125, and HCG to better understand their diagnostic and prognostic features.
Methods
Type of study: Cross-sectional
Study design: Retrospective and prospective
Study duration: A 4-year study conducted in the Department of Pathology at SVS Medical College, Mahabubnagar, Telangana, India, within January 2019 to August 2023
Sample size: 50 cases (Age range: 11-70 years)
Sample size calculation: Prevalence of (p) = 3.9%
Sample size estimation
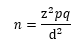
Breast cancer (BC) is the most common malignancy in women worldwide and accounts for approximately 1,000,000 mortalities annually (1). In India, ovarian tumors rank as the fifth leading cause of cancer-related mortality among women (2). Breast cancer represents 6% of all cancers in Indian women, with an annual prevalence of 9 per 100,000 individuals (3). Most breast tumors occur in women aged 25-45 years. Established risk factors include positive family history, genetic mutations, and hormonal imbalances (4). The majority of breast carcinomas originate from the epithelial lining of the ducts or lobules; accordingly, carcinomas are classified as ductal or lobular carcinomas. Histologic grading reflects the degree of differentiation, indicating the resemblance of tumor cells to normal breast tissue (5).
Ovarian tumorigenesis is thought to result from repeated endocrine and mechanical trauma during monthly ovulatory cycles. The cumulative effect of ovulatory rupture and repair might contribute to genetic mutations leading to malignancy. This mechanism explains the protective effects of oral contraceptive use, late menarche, early menopause, multiparity, and breastfeeding, all of which reduce the number of ovulatory cycles (6). Ultrasonography and cancer antigen 125 (CA-125) assessments serve as screening tools for early detection; however, Fine Needle Aspiration Cytology (FNAC) provides primary diagnosis and prognostic information with 90-95% accuracy in differentiating benign from malignant lesions (7). According to the World Health Organization (WHO) guidelines, ovarian tumors are histologically classified as benign, borderline, or malignant, with this classification carrying significant therapeutic and prognostic implications. Variations in histopathological patterns are commonly observed in both primary and secondary ovarian tumors (8).
In BC, ongoing studies are utilizing panels of antibodies, such as estrogen receptor (ER), progesterone receptor (PR), human EGF receptor-2 (HER2)/neu, cytokeratin 5/6, epidermal growth factor receptor (EGFR), and Ki-67, to classify tumors into molecular subgroups (9). Similarly, in ovarian germ cell tumors, alpha-fetoprotein (AFP) is associated with yolk sac elements; however, human chorionic gonadotropin (HCG) serves as a marker of trophoblastic differentiation.
The present study aimed to investigate the histomorphological spectrum and histological grading of ovarian germ cell tumors using the modified Bloom-Richardson Grading System. Furthermore, this study sought to molecularly classify these tumors using AFP, CA-125, and HCG to better understand their diagnostic and prognostic features.
Methods
Type of study: Cross-sectional
Study design: Retrospective and prospective
Study duration: A 4-year study conducted in the Department of Pathology at SVS Medical College, Mahabubnagar, Telangana, India, within January 2019 to August 2023
Sample size: 50 cases (Age range: 11-70 years)
Sample size calculation: Prevalence of (p) = 3.9%
Sample size estimation
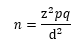
- Z = 1.96 ≈ 2 (Considering confidence as 95%)
- p = prevalence (Considered 15% as the exact prevalence is not known)
- q = 100 - p, that is 85%
- d = Absolute error, which was 10%
- n = 2 × 2 × 0.15 × 0.85/0.1 × 0.1
- n = 51
Therefore, the study was conducted on 50 patients.
Inclusion criteria
All cases of germ cell ovarian tumors
Exclusion criteria
Inflammatory conditions, other ovarian tumors, and metastasis
A thorough medical history was taken, including information on menstruation, age, gender, and family history.
Procedure
Immunohistochemistry (IHC) was performed using markers, including AFP, CA-125, and HCG. Tissue samples were first processed and embedded in paraffin wax blocks. Three sections, each 3-4 μm thick, were prepared for hematoxylin and eosin (H and E) staining. For immunohistochemical analysis, the sections were mounted on poly-L-lysine-coated slides. Monoclonal antibodies targeting AFP, CA-125, and HCG (Dako Ready-to-Use primary antibodies) were applied to formalin-fixed, paraffin-embedded (FFPE) tissue sections according to the manufacturer’s protocol.
The data were collected and compiled, including patient age, clinical presentation, and detailed histopathological findings of the tissue sections, to support the accurate diagnosis and further analysis of the germ cell tumors.
Results
In this study, the majority of patients with ovarian tumors were over 31 years, with a mean age of 39.5 ± 6.9 years. The most common presenting symptom was an abdominal mass in 28 patients (56%), followed by abdominal pain in 10 patients (20%). Key characteristics, including age distribution, presenting symptoms, tumor consistency, and malignancy status, are summarized in Table 1.
Figure 1 illustrates the imaging findings and H&E staining of ovarian teratomas. Figure 2 depicts the histopathological features of embryonal carcinoma, dysgerminoma, and choriocarcinoma, along with their H and E staining interpretations.
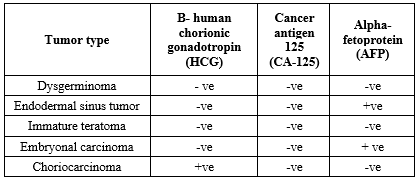
The histopathological spectrum of germ cell ovarian tumors, classified according to WHO guidelines, is detailed in Table 2. Figure 3 illustrates immunohistochemical staining results, including HCG positivity in choriocarcinoma, AFP expression in embryonal carcinoma, and AFP expression in endodermal sinus tumor cells.
HCG-/CA125-/AFP+ expression was also observed in embryonal carcinoma, whereas HCG+/CA125-/AFP- expression was noted in choriocarcinoma (Table 3).
Inclusion criteria
All cases of germ cell ovarian tumors
Exclusion criteria
Inflammatory conditions, other ovarian tumors, and metastasis
A thorough medical history was taken, including information on menstruation, age, gender, and family history.
Procedure
Immunohistochemistry (IHC) was performed using markers, including AFP, CA-125, and HCG. Tissue samples were first processed and embedded in paraffin wax blocks. Three sections, each 3-4 μm thick, were prepared for hematoxylin and eosin (H and E) staining. For immunohistochemical analysis, the sections were mounted on poly-L-lysine-coated slides. Monoclonal antibodies targeting AFP, CA-125, and HCG (Dako Ready-to-Use primary antibodies) were applied to formalin-fixed, paraffin-embedded (FFPE) tissue sections according to the manufacturer’s protocol.
The data were collected and compiled, including patient age, clinical presentation, and detailed histopathological findings of the tissue sections, to support the accurate diagnosis and further analysis of the germ cell tumors.
Results
In this study, the majority of patients with ovarian tumors were over 31 years, with a mean age of 39.5 ± 6.9 years. The most common presenting symptom was an abdominal mass in 28 patients (56%), followed by abdominal pain in 10 patients (20%). Key characteristics, including age distribution, presenting symptoms, tumor consistency, and malignancy status, are summarized in Table 1.
Figure 1 illustrates the imaging findings and H&E staining of ovarian teratomas. Figure 2 depicts the histopathological features of embryonal carcinoma, dysgerminoma, and choriocarcinoma, along with their H and E staining interpretations.
The histopathological spectrum of germ cell ovarian tumors, classified according to WHO guidelines, is detailed in Table 2. Figure 3 illustrates immunohistochemical staining results, including HCG positivity in choriocarcinoma, AFP expression in embryonal carcinoma, and AFP expression in endodermal sinus tumor cells.
HCG-/CA125-/AFP+ expression was also observed in embryonal carcinoma, whereas HCG+/CA125-/AFP- expression was noted in choriocarcinoma (Table 3).
|
Table 1. Age distribution, symptoms, consistency of the tumors, and malignancy of patients with ovarian cancer
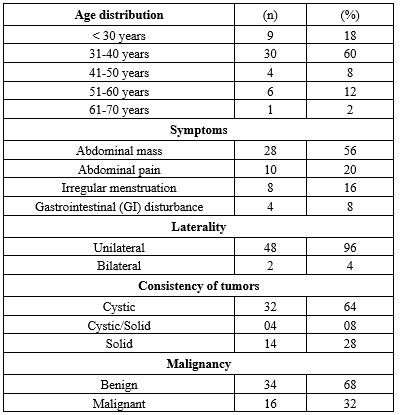 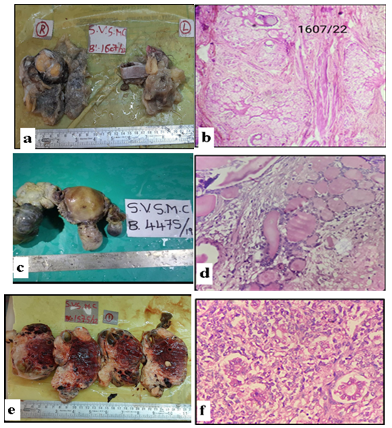 Figure 1. Hematoxylin and eosin (H and E) staining; a) Gross image of teratoma of ovary; b) Teratoma of ovary (Mature)- H and E staining (400×); c) Gross image of struma ovarii; d) Struma ovarii- H and E staining (400×); e) Endodermal sinus tumor (Gross image); f) Endodermal sinus tumor- H and E staining (400×) |
.PNG) Figure 2. Hematoxylin and eosin (H and E) staining; a) Gross image of embryonal carcinoma; b) Embryonal carcinoma- H and E staining (Magnification: 400×); c) Gross image of dysgerminoma; d) Dysgerminoma- H and E staining (Magnification: 400×); e) Gross image of choriocarcinoma; f) Choriocarcinoma- H and E staining (Magnification: 400×) Table 2. Histopathological spectrum of germ cell ovarian tumors as per World Health Organization (WHO) classification .PNG) Abbreviations: WHO: World Health Organization .PNG) Figure 3. Immunohistochemistry; a and b) Human chorionic gonadotropin (HCG) positive in choriocarcinoma (Magnification: 400×); c) Alpha-fetoprotein (AFP) positive in embryonal carcinoma (Magnification: 100×); d) AFP positive in endodermal sinus tumor (Magnification: 100×); e) AFP negative (Magnification: 400×); f) Cancer antigen 125 (CA-125) negative (Magnification: 400×) |
Discussion
Ovarian tumors occur most commonly in women of reproductive age, with approximately two-thirds of cases diagnosed in the aforementioned group (10). These tumors often remain clinically silent due to their deep anatomical location, with abdominal mass and pain being the most frequent presenting symptoms. In the present study, the patients were within the age range of 11-70 years, with a mean age of 33 years. The youngest patient was an 11-year-old female with a unilateral mature teratoma (9.5 × 5.5 × 4 cm) presenting with abdominal pain, the most common germ cell tumor in adolescents. The oldest patient was a 70-year-old female with unilateral mucinous cystadenocarcinoma (19 × 14 × 12 cm) presenting with ascites. The majority of cases (77.78%) were within the age range of 21–50 years, consistent with the findings of previous studies (11,12).
Premenopausal women showed a higher prevalence of ovarian tumors, whereas postmenopausal women exhibited a relatively higher prevalence of malignant tumors, consistent with the findings reported by Jha et al. and Kayastha et al. (11,12). The tumors were sometimes incidentally detected on ultrasound; however, symptomatic patients were categorized based on the predominant presenting complaint. In the present study, the most frequent symptom was an abdominal mass (56%), followed by abdominal pain (20%) and menstrual irregularities, including postmenopausal bleeding (16%). The aforementioned results align with the findings of prior studies by Rashid et al. and Jamal et al., which reported abdominal pain and mass as the most common presenting complaints (13,14).
Most tumors in the present cohort were unilateral (96%), with only 4% presenting bilaterally; among bilateral tumors, malignancy was more frequent. The obtained result is similar to the findings reported by Jha et al. (11) and Tushar et al. (15), although the prevalence of bilaterality varied among studies. Tumor sizes ranged from 3 to 30 cm, with 88% measuring less than 20 cm. The largest tumor was a 30 × 25 × 15 cm unilateral benign papillary serous cystadenofibroma, comparable to Pilli et al.’s observations.
Regarding architecture, 64%, 28%, and 8% of the tumors were purely cystic, solid, and mixed, respectively. These proportions are consistent with the findings of Kar et al.; however, differences might be explained by a higher number of malignant tumors in the aforementioned study. Solid or complex tumors were associated with higher malignancy risk, as shown by Timmerman et al. (16). Overall, 68% and 32% of the tumors were benign and malignant, respectively, with benign tumors being predominantly cystic and malignant tumors solid or mixed, in line with the findings of Gupta et al. (17), Madan et al. (18), and Choudhary et al. (19).
Germ cell tumors were classified as benign or malignant based on predominant cell type, growth pattern, stromal content, and cellular atypia with invasiveness. Immunohistochemistry revealed HCG-/CA125-/AFP- expression in dysgerminomas and immature teratomas; nevertheless, endodermal sinus tumors showed HCG-/CA125-/AFP+ expression. The frequencies of benign (68%) and malignant (32%) germ cell tumors were comparable to those of previous studies by Mandal et al. (Benign: 63.1%, malignant: 29.6%) and Chavan et al. (Benign: 70.1%, malignant: 29.9%) (20,21).
In the current study, age was a significant factor; accordingly, the tumors in patients under 40 years were mostly benign, whereas the tumors in patients over 40 showed a higher prevalence of malignancy. Malignant tumors in younger adults were primarily germ cell or mixed germ cell tumors.
Study limitations
The current study was limited by a small sample size, which restricted broader analysis.
Conclusion
The increased prevalence of malignancy was observed in patients over 50 years of age, in the postmenopausal group, in tumors with solid or complex morphology, and in bilateral tumors. The aforementioned parameters might serve as useful predictors of malignancy in ovarian tumors. Among ovarian neoplasms, germ cell tumors are relatively uncommon. Benign tumors are primarily mature cystic teratomas; however, malignant germ cell tumors include dysgerminomas. Choriocarcinomas demonstrate strong HCG positivity, whereas endodermal sinus tumors and embryonal carcinomas show strong AFP expression.
Acknowledgement
None.
Funding sources
Not received any funding.
Ethical statement
This study received approval from the Ethics Committee of SVS Medical College, under an approval code (SVS/IEC/2023/Path/03).
Conflicts of interest
The authors declared no conflict of interest.
Author contributions
DS, SB, SK, SAB, PRL, and SS collected the data. RF designed the protocol and conceptualized the study. All authors revised and analyzed the data. All authors worked together in drafting the manuscript. The final version of the draft was reviewed and approved by all authors.
Data availability statement
Data is available upon request from the corresponding author.
Article Type: Research |
Subject:
Cellular and Molecular Biology
References
1. Mondal SK, Banyopadhyay R, Nag DR, Roychowdhury S, Mondal PK, Sinha SK. Histologic pattern, bilaterality and clinical evaluation of 957 ovarian neoplasms: a 10-year study in a tertiary hospital of eastern India. J Cancer Res Ther. 2011;7(4):433-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Roth LM. Recent advances in the pathology and classification of ovarian sex cord-stromal tumors. Int J Gynecol Pathol. 2006;25(3):199-215. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Hankinson SE, Danforth KN. Ovarian cancer. In: Schottenfeld D, Fraumeni JF, eds. Cancer Epidemiology and Prevention. 3rd ed. New York(NY): Oxford University Press; 2006. p. 1013-26. [View at Publisher] [DOI] [Google Scholar]
4. Thomas J, Nachimuthu N, James C, Barbara K, Swede H. Risk factors for invasive epithelial ovarian cancer by histologic subtype. Online J Health Allied Sci. 2004;3(3). [View at Publisher] [Google Scholar]
5. Pilli GS, Suneeta KP, Dhaded AV, Yenni VV. Ovarian tumours: a study of 282 cases. J Indian Med Assoc. 2002;100(7):420-3. [View at Publisher] [PMID] [Google Scholar]
6. Chen LM, Karlan BY. Early detection and risk reduction for familial gynecologic cancers. Clin Obstet Gynecol. 1998;41(1):200-14. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Kurman RJ, Visvanathan K, Roden R, Wu TC, Shih IM. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol. 2008;198(4):351-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Lee KR, Young RH. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings of 50 cases. Am J Surg Pathol. 2003;27(3):281-92. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Norris HJ, Jensen RD. Relative frequency of ovarian neoplasms in children and adolescents. Cancer. 1972;30(3):713-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Rosai J. Rosai and Ackerman's surgical pathology. 9th ed. St. Louis: CV Mosby Co.; 2004. Vol. 2. p. 1649-736. [View at Publisher] [Google Scholar]
11. Jha R, Karki S. Histological pattern of ovarian tumors and their age distribution. Nepal Med Coll J. 2008;10(2):81-5. [View at Publisher] [Google Scholar]
12. Kayastha S. Study of ovarian tumors in Nepal Medical College Teaching Hospital. Nepal Med Coll J. 2009;11(3):200-2. [PMID]
13. Rashid S, Sarwar G, Ali A. A clinic pathological study of ovarian cancer. Mother-Child. 1998;36(4):117-95. [View at Publisher]
14. Jagan A, Sasikala, Dilshath. A clinic pathological study of ovarian Tumor: A prospective study in a tertiary care hospital south India. Int J Clin Obstet Gynaecol. 2020;4(6):01-05. [View at Publisher] [DOI]
15. Tushar K, Asanranthi K, Mohapatra PC. Intraoperative cytology of ovarian tumors. J Obstet Gynecol India. 2005;55(4):345-49. [View at Publisher] [Google Scholar]
16. Timmerman D, Van Calster B, Testa A, Savelli L, Fischerova D, Froyman W, et al. Predicting the risk of malignancy in adnexal masses based on the Simple Rules from the International Ovarian Tumor Analysis group. Am J Obstet Gynecol. 2016;214(4):424-37. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Gupta N, Bisht D, Agarwal AK, Sharma VK. Retrospective and Prospective Study of Ovarian Tumours and Tumour Like Lesions. India J Pathol Microbial. 2007;50(3):525-27. [View at Publisher] [PMID] [Google Scholar]
18. Madan A, Tyagi SP, Mohsin S, Hameed F, Rizvi R. Incidence of Ovarian Tumours at Aligarh with Particular Reference to Histopathological Typing. J Obstet Gynecol India. 1978;827-32. [View at Publisher]
19. Choudhary M, Yadav S, Kumar R, Chaudhary R. HISTOPATHOLOGICAL SPECTRUM OF OVARIAN TUMOURS WITH DIAGNOSTIC ACCURACY OF SERUM CA125 AND HE4. Int J Acad Med Pharm. 2024;6(3):28-32. [View at Publisher] [Google Scholar]
20. Mondal SK, Banyopadhyay R, Nag DR, Roychowdhury S, Mondal PK, Sinha SK. Histologic pattern, bilaterality and clinical evaluation of 957 ovarian neoplasms: A 10-year study in a tertiary hospital of eastern India. J Cancer Res Ther. 2011;7(4):433-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Chavan SS, Toppo SM. Study of 92 cases of ovarian germ cell tumour at tertiary health care centre. Indian J Pathol Oncol. 2019;6(3):464-70. [View at Publisher] [DOI] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |