Volume 8, Issue 3 (Journal of Clinical and Basic Research (JCBR) 2024)
jcbr 2024, 8(3): 1-4 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
sharma S, Kacker S, Saboo N. Exploring patterns of heart rate variability in major depressive disorder: A short-term study in Jaipur, Rajasthan. jcbr 2024; 8 (3) :1-4
URL: http://jcbr.goums.ac.ir/article-1-462-en.html
URL: http://jcbr.goums.ac.ir/article-1-462-en.html
1- Department of Physiology, RUHS College of Medical Sciences, Jaipur, India
2- Department of Physiology, RUHS College of Medical Sciences, Jaipur, India ,nehasaboo8@gmil.com
2- Department of Physiology, RUHS College of Medical Sciences, Jaipur, India ,
Full-Text [PDF 497 kb]
(720 Downloads)
| Abstract (HTML) (2095 Views)
Results
Figure 1 depicts the distribution of participants by demographic variable, highlighting the dominance of specific categories. It demonstrates that most participants were from rural areas, aged 20-30, married, and belonged to the upper middle class. Furthermore, males were slightly more prevalent (45) compared to females (40).
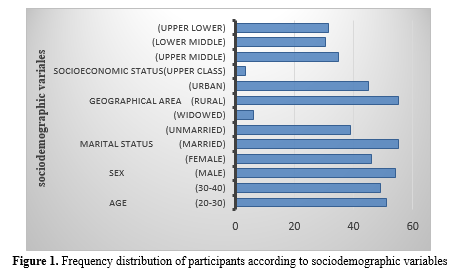
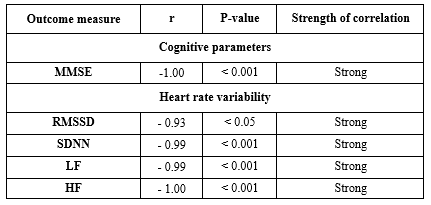
Table 1 depicts correlations between HAM-D (Hamilton Depression Rating Scale) scores and physiological and cognitive measures. The Mini-Mental State Examination (MMSE) scores and HAM-D scores have a perfect negative association (r = -1.00, p < 0.001), which suggests that with an increase in depression severity, cognitive performance decreases as assessed by the MMSE. Similarly, there was a strong negative correlation between HAM-D scores and the following variables: LF (r = -0.99, p < 0.001), SDNN (r = -0.99, p < 0.01), RMSSD (r = -0.93, p < 0.01), and mean heart rate (r = -0.99, p < 0.001). These results indicate that lower heart rate variability, as seen by lower RMSSD, SDNN, and LF values, as well as a lower mean heart rate, are linked to higher depression scores. On the other hand, there was a highly significant and negative correlation (r = -1.00, p < 0.001) between HAM-D scores and HF (High-frequency power), which highlights the effect of depression on autonomic nervous system function.
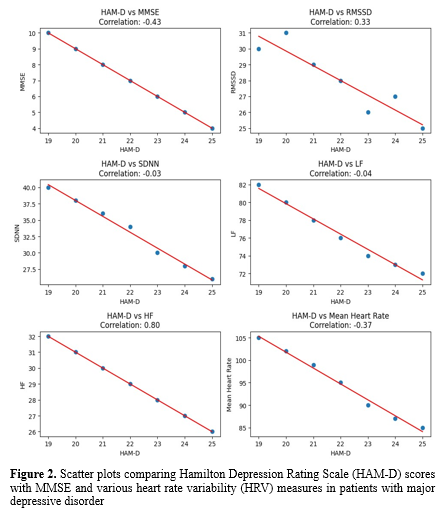
Figure 2 shows the correlation analysis between HAMD, MMSE, and heart rate variability parameters (RMSSD): Root Mean Square of the Successive Difference between normal heartbeats, PNN50: Percentage of the number of pairs of NN that is longer than 50, SDNN: Standard Deviation of NN interval, LF: Low Frequency, HF: High Frequency, Mean Heart Rate (mean HR).
Discussion
This study examined the relationships between depression severity, as measured by the Hamilton Depression Rating Scale (HAM-D), and various heart rate variability (HRV) parameters in patients with major depressive disorder (MDD).
The most notable observation was a significant positive connection between HAM-D scores and high-frequency (HF) power (r = 0.80). This finding contradicts the common notion that depression is related to decreased HF power, which is often interpreted as decreased parasympathetic activity (18). Kemp et al. (2010) observed that patients with major depressive disorder (MDD) exhibited lower HF power compared to healthy controls in a meta-analysis (19). Our findings indicate that in our sample, parasympathetic activity increased with depression severity, which is consistent with the findings of the study conducted by Singla et al. (20).
This study's weak positive connection between HAM-D scores and RMSSD (r = 0.33) is consistent with the HF power finding, as both are considered measures of parasympathetic activity. However, it contradicts prior research that reported lower RMSSD in depression (21). The absence of association between HAM-D and SDNN (r = -0.03) shows that depression severity may not have significantly affected the overall HRV in our population, contrary to some earlier studies (22).
Interestingly, no significant association was found between HAM-D ratings and low-frequency (LF) power (r = -0.04). This contradicts previous research that reported lower LF power in depression (19). The lack of a relationship in our study might suggest that depression severity did not systematically affect our group's sympathetic component of HRV.
The moderate negative correlation (r = -0.43) between HAM-D and MMSE scores supports the previously documented link between depression and cognitive function (23). This conclusion is consistent with the expanding body of research indicating a bidirectional link between depression and cognitive decline (24).
The slight negative correlation between HAM-D ratings and mean heart rate (r = -0.37) was entirely unexpected, given that depression has frequently been linked to increased heart rate (25). This conclusion may reflect the complicated interplay between depression and autonomic regulation, which could be altered by medication or concomitant diseases. In contrast, there is evidence indicating that there is no difference in HRV between individuals with depression and healthy individuals (26).
Heart rate variability (HRV) in major depression is caused by various interconnected systems, including the autonomic nervous system, the hypothalamic-pituitary-adrenal (HPA) axis, neurotransmitter imbalances, inflammation, and behavioral variables. Depression is frequently characterized by an overactive sympathetic nervous system and diminished parasympathetic (Vagal) tone, resulting in lower HF HRV. Dysregulation of the HPA axis causes high-stress hormones such as cortisol, further suppressing parasympathetic function. Neurotransmitter abnormalities, particularly those involving serotonin, norepinephrine, and GABA, contribute to autonomic dysregulation. Genetic and epigenetic factors can make a person more susceptible to depression and autonomic dysfunction, which in turn can further decrease heart rate variability (HRV) (27). However, as stated by Porges et al., the vagus nerve regulates heart rate by slowing it down via its influence on the heart pacemaker. This modulation is essential for maintaining a healthy autonomic nervous system. Low vagal tone, which is common in depression, reduces HRV and increases the risk of heart disease. The vagus nerve regulates heart rate, which is crucial for mental and physical well-being (28).
This study had significant limitations. Multiple investigations have demonstrated that antidepressants influence HRV indicators (12,26), while this study provided no information regarding the medications the patients used. This can be considered a significant study limitation since medication use by patients could have influenced the outcomes. Moreover, there was no healthy control group in this study. Furthermore, we excluded cardiac diseases such as ischemic heart disease and myocardial infarction as they can be confounding factors.
The use of standardized tools to screen for comorbid depression in participants, together with the administration of psychological test scales and HRV measures at the initial visit, can be considered as the advantages of this study. Additionally, since HRV was measured before treatment, a more precise analysis of the correlation between depression symptoms and HRV indices was possible. This can be crucial as HRV can be pretty sensitive to environmental factors. To ensure consistency in measurement and result interpretation, the same inspector conducted all tests and measured HRV at the same place throughout the study. To the best of our knowledge, research on the connection between depression and HRV is still sparse. This study's clinical implications include the potential use of the findings as significant biomarkers for the assessment and management of individuals with depression at an earlier age.
Conclusion
In this study, we discovered a positive correlation between greater feelings of depression and increased parasympathetic activation. We found a significant negative connection between HAM-D scores, heart rate, and Mini-Mental State Examination (MMSE) scores, suggesting that severe depressive symptoms are associated with lower heart rates and poorer cognitive function. These findings show the complex and multidimensional links between depressive symptoms, autonomic function, and cognitive performance, emphasizing the need for a comprehensive approach to understanding and treating major depression. HRV can be considered an essential potential biomarker to assess cardiovascular health in the early stages of life.
Acknowledgement
We would like to thank all the participants who gave consent to participate in this study, the faculty of the Department of Physiology, and all hospital staff.
Funding sources
None.
Ethical statement
Institutional Ethical approval was obtained from the Institutional Ethics Committee (RUHS-CMS/Ethics Comm./2022/70). Each participant provided informed consent to participate in the study.
Conflicts of interest
There are no conflicts of interest.
Author contributions
SS analyzed and interpreted the data regarding subjects. NS also interpreted the data and was a significant contributor to the manuscript. SK drafted the work or substantively revised it. All authors read and approved the final manuscript.
Full-Text: (558 Views)
Introduction
Depression is a prevalent but serious mood disorder (Sometimes known as a major depressive disorder or clinical depression). Severe symptoms impact mood, cognition, and day-to-day functioning, including eating, sleeping, and working (1).
In 2015, it was projected that over 300 million individuals worldwide suffered from depression, making up about 4.3% of the world's population. Major depressive disorder (DD) ranked as the third most common cause of disability in 2015, with an increasing global burden of depression. (2) The estimated prevalence of depressive episode/DD worldwide is between 3.2% and 4.7% (3,4). The higher risk associated with depression may be explained by the fact that depression is also connected to increased HR and lower HRV, both of which are established risk factors for cardiac morbidity and death (5).
This reduction in HRV often indicates an imbalance in the autonomic nervous system, with a dominance of sympathetic (Fight-or-flight) activity and a decrease in parasympathetic (Rest-and-digest) activity (6). Reduced HRV in MDD is associated with a range of clinical symptoms, including increased stress, anxiety, and a higher risk of cardiovascular problems. Researchers have identified HRV as a potential biomarker for MDD. It can be used to assess the severity of depressive symptoms and may help in predicting the risk of future depressive episodes. Abnormal HRV can be linked to specific symptoms of MDD, such as anhedonia (Loss of interest in pleasurable activities) and altered emotional regulation (7,8). The exact mechanisms underlying reduced HRV in MDD are not fully understood. Still, it is believed to be related to altered autonomic nervous system functioning, inflammation, and disruptions in the regulation of the heart rate. HRV is acknowledged as a valuable and non-invasive method for assessing how the heart's autonomic nervous system is regulated (9). The variation between successive heartbeats is referred to as HRV. The autonomic nervous system's sympathetic and parasympathetic branches work together to regulate the SA node, which regulates the heart's rhythm. HR tends to rise in response to sympathetic activity very slowly (A few seconds). Conversely, parasympathetic activity mediates quickly (0.2-0.6 s) (10) and tends to lower heart rate. In physically healthy depressed adults, HRV does not differ from that of healthy controls, according to one study by Sayar K et al. (11), while Licht et al. demonstrated a substantial correlation between depression and reduced HRV (12).
Therefore, this study aimed to demonstrate the status of heart rate variability parameters in a group with major depressive disorder at a specialized care facility.
Methods
Study design and participants: This observational study spanned six months and involved the enrollment of 90 patients aged 20-40 years diagnosed with major depressive disorder (MDD) as per ICD-10 criteria (13) at the outpatient psychiatric department. Exclusion criteria included psychiatric illnesses, including schizophrenia or schizoaffective disorder and bipolar disorder. Besides, those with organic disorders such as dementia, epilepsy, cerebrovascular diseases, history of electroconvulsive therapy in the last three months, musculoskeletal disorders such as kyphosis, scoliosis, and chronic diseases such as hypertension, diabetes, and chronic renal disease were excluded.
Ethics and consent: The study protocol was approved by Institutional Ethical Number RUHS-CMS/Ethics Comm./2022/70. Before enrollment, all participants were provided with a participation information sheet, and written informed consent was obtained.
Data collection: The Hamilton Rating Scale for Depression (14) was used to assess major depressive disorders after recruitment from the psychiatry department.
Depression is a prevalent but serious mood disorder (Sometimes known as a major depressive disorder or clinical depression). Severe symptoms impact mood, cognition, and day-to-day functioning, including eating, sleeping, and working (1).
In 2015, it was projected that over 300 million individuals worldwide suffered from depression, making up about 4.3% of the world's population. Major depressive disorder (DD) ranked as the third most common cause of disability in 2015, with an increasing global burden of depression. (2) The estimated prevalence of depressive episode/DD worldwide is between 3.2% and 4.7% (3,4). The higher risk associated with depression may be explained by the fact that depression is also connected to increased HR and lower HRV, both of which are established risk factors for cardiac morbidity and death (5).
This reduction in HRV often indicates an imbalance in the autonomic nervous system, with a dominance of sympathetic (Fight-or-flight) activity and a decrease in parasympathetic (Rest-and-digest) activity (6). Reduced HRV in MDD is associated with a range of clinical symptoms, including increased stress, anxiety, and a higher risk of cardiovascular problems. Researchers have identified HRV as a potential biomarker for MDD. It can be used to assess the severity of depressive symptoms and may help in predicting the risk of future depressive episodes. Abnormal HRV can be linked to specific symptoms of MDD, such as anhedonia (Loss of interest in pleasurable activities) and altered emotional regulation (7,8). The exact mechanisms underlying reduced HRV in MDD are not fully understood. Still, it is believed to be related to altered autonomic nervous system functioning, inflammation, and disruptions in the regulation of the heart rate. HRV is acknowledged as a valuable and non-invasive method for assessing how the heart's autonomic nervous system is regulated (9). The variation between successive heartbeats is referred to as HRV. The autonomic nervous system's sympathetic and parasympathetic branches work together to regulate the SA node, which regulates the heart's rhythm. HR tends to rise in response to sympathetic activity very slowly (A few seconds). Conversely, parasympathetic activity mediates quickly (0.2-0.6 s) (10) and tends to lower heart rate. In physically healthy depressed adults, HRV does not differ from that of healthy controls, according to one study by Sayar K et al. (11), while Licht et al. demonstrated a substantial correlation between depression and reduced HRV (12).
Therefore, this study aimed to demonstrate the status of heart rate variability parameters in a group with major depressive disorder at a specialized care facility.
Methods
Study design and participants: This observational study spanned six months and involved the enrollment of 90 patients aged 20-40 years diagnosed with major depressive disorder (MDD) as per ICD-10 criteria (13) at the outpatient psychiatric department. Exclusion criteria included psychiatric illnesses, including schizophrenia or schizoaffective disorder and bipolar disorder. Besides, those with organic disorders such as dementia, epilepsy, cerebrovascular diseases, history of electroconvulsive therapy in the last three months, musculoskeletal disorders such as kyphosis, scoliosis, and chronic diseases such as hypertension, diabetes, and chronic renal disease were excluded.
Ethics and consent: The study protocol was approved by Institutional Ethical Number RUHS-CMS/Ethics Comm./2022/70. Before enrollment, all participants were provided with a participation information sheet, and written informed consent was obtained.
Data collection: The Hamilton Rating Scale for Depression (14) was used to assess major depressive disorders after recruitment from the psychiatry department.
- Sociodemographic detail was recorded according to the modified Kuppuswamy Scale 2021(Sex, marital status, geographical area, socioeconomic status) (15).
- Cognitive functions were assessed using the MMSE (Mini-Mental Status Examination) (16) questionnaire.
- Autonomic functions were assessed by heart rate variability indices (17), including:
- Time domain (RMSSD: Root Mean Square of the Successive Difference between normal heartbeats, PNN50: Percentage of number of pairs of NN that is longer than 50, SDNN: Standard Deviation of NN interval)
- Frequency domain parameters (LF: Low Frequency, HF: High Frequency, LF/HF: Low-Frequency/High-Frequency ratio) were recorded using a digital physiograph (MLT004/ST) by AD instruments (The electrocardiogram or ECG) which can be recorded for five minutes to determine HRV. The ECG extracted intervals between successive beats or R-R intervals. Rather than using the ECG, the peripheral pulse was used in this experiment to make capturing a signal for analysis easier. A peripheral pulse occurs during each normal cardiac cycle. Therefore, the pulse's peak-to-peak interval corresponded to the R-R interval from an ECG recording. This technique involves studying and showing the various frequency components of the N-N intervals.
Statistical analysis: All analyses were conducted using Microsoft Excel 2019 and analyzed using SPSS 21.
- Descriptive statistics were calculated for all variables and presented as mean and standard deviation.
- Pearson's or Spearman's correlations were used to examine relationships between MMSE, HRV measures, and depression severity scores.
Results
Figure 1 depicts the distribution of participants by demographic variable, highlighting the dominance of specific categories. It demonstrates that most participants were from rural areas, aged 20-30, married, and belonged to the upper middle class. Furthermore, males were slightly more prevalent (45) compared to females (40).

Table 1 depicts correlations between HAM-D (Hamilton Depression Rating Scale) scores and physiological and cognitive measures. The Mini-Mental State Examination (MMSE) scores and HAM-D scores have a perfect negative association (r = -1.00, p < 0.001), which suggests that with an increase in depression severity, cognitive performance decreases as assessed by the MMSE. Similarly, there was a strong negative correlation between HAM-D scores and the following variables: LF (r = -0.99, p < 0.001), SDNN (r = -0.99, p < 0.01), RMSSD (r = -0.93, p < 0.01), and mean heart rate (r = -0.99, p < 0.001). These results indicate that lower heart rate variability, as seen by lower RMSSD, SDNN, and LF values, as well as a lower mean heart rate, are linked to higher depression scores. On the other hand, there was a highly significant and negative correlation (r = -1.00, p < 0.001) between HAM-D scores and HF (High-frequency power), which highlights the effect of depression on autonomic nervous system function.
|
Table 1. Association of HAM-D with cognitive and heart rate variability parameters
 |

Discussion
This study examined the relationships between depression severity, as measured by the Hamilton Depression Rating Scale (HAM-D), and various heart rate variability (HRV) parameters in patients with major depressive disorder (MDD).
The most notable observation was a significant positive connection between HAM-D scores and high-frequency (HF) power (r = 0.80). This finding contradicts the common notion that depression is related to decreased HF power, which is often interpreted as decreased parasympathetic activity (18). Kemp et al. (2010) observed that patients with major depressive disorder (MDD) exhibited lower HF power compared to healthy controls in a meta-analysis (19). Our findings indicate that in our sample, parasympathetic activity increased with depression severity, which is consistent with the findings of the study conducted by Singla et al. (20).
This study's weak positive connection between HAM-D scores and RMSSD (r = 0.33) is consistent with the HF power finding, as both are considered measures of parasympathetic activity. However, it contradicts prior research that reported lower RMSSD in depression (21). The absence of association between HAM-D and SDNN (r = -0.03) shows that depression severity may not have significantly affected the overall HRV in our population, contrary to some earlier studies (22).
Interestingly, no significant association was found between HAM-D ratings and low-frequency (LF) power (r = -0.04). This contradicts previous research that reported lower LF power in depression (19). The lack of a relationship in our study might suggest that depression severity did not systematically affect our group's sympathetic component of HRV.
The moderate negative correlation (r = -0.43) between HAM-D and MMSE scores supports the previously documented link between depression and cognitive function (23). This conclusion is consistent with the expanding body of research indicating a bidirectional link between depression and cognitive decline (24).
The slight negative correlation between HAM-D ratings and mean heart rate (r = -0.37) was entirely unexpected, given that depression has frequently been linked to increased heart rate (25). This conclusion may reflect the complicated interplay between depression and autonomic regulation, which could be altered by medication or concomitant diseases. In contrast, there is evidence indicating that there is no difference in HRV between individuals with depression and healthy individuals (26).
Heart rate variability (HRV) in major depression is caused by various interconnected systems, including the autonomic nervous system, the hypothalamic-pituitary-adrenal (HPA) axis, neurotransmitter imbalances, inflammation, and behavioral variables. Depression is frequently characterized by an overactive sympathetic nervous system and diminished parasympathetic (Vagal) tone, resulting in lower HF HRV. Dysregulation of the HPA axis causes high-stress hormones such as cortisol, further suppressing parasympathetic function. Neurotransmitter abnormalities, particularly those involving serotonin, norepinephrine, and GABA, contribute to autonomic dysregulation. Genetic and epigenetic factors can make a person more susceptible to depression and autonomic dysfunction, which in turn can further decrease heart rate variability (HRV) (27). However, as stated by Porges et al., the vagus nerve regulates heart rate by slowing it down via its influence on the heart pacemaker. This modulation is essential for maintaining a healthy autonomic nervous system. Low vagal tone, which is common in depression, reduces HRV and increases the risk of heart disease. The vagus nerve regulates heart rate, which is crucial for mental and physical well-being (28).
This study had significant limitations. Multiple investigations have demonstrated that antidepressants influence HRV indicators (12,26), while this study provided no information regarding the medications the patients used. This can be considered a significant study limitation since medication use by patients could have influenced the outcomes. Moreover, there was no healthy control group in this study. Furthermore, we excluded cardiac diseases such as ischemic heart disease and myocardial infarction as they can be confounding factors.
The use of standardized tools to screen for comorbid depression in participants, together with the administration of psychological test scales and HRV measures at the initial visit, can be considered as the advantages of this study. Additionally, since HRV was measured before treatment, a more precise analysis of the correlation between depression symptoms and HRV indices was possible. This can be crucial as HRV can be pretty sensitive to environmental factors. To ensure consistency in measurement and result interpretation, the same inspector conducted all tests and measured HRV at the same place throughout the study. To the best of our knowledge, research on the connection between depression and HRV is still sparse. This study's clinical implications include the potential use of the findings as significant biomarkers for the assessment and management of individuals with depression at an earlier age.
Conclusion
In this study, we discovered a positive correlation between greater feelings of depression and increased parasympathetic activation. We found a significant negative connection between HAM-D scores, heart rate, and Mini-Mental State Examination (MMSE) scores, suggesting that severe depressive symptoms are associated with lower heart rates and poorer cognitive function. These findings show the complex and multidimensional links between depressive symptoms, autonomic function, and cognitive performance, emphasizing the need for a comprehensive approach to understanding and treating major depression. HRV can be considered an essential potential biomarker to assess cardiovascular health in the early stages of life.
Acknowledgement
We would like to thank all the participants who gave consent to participate in this study, the faculty of the Department of Physiology, and all hospital staff.
Funding sources
None.
Ethical statement
Institutional Ethical approval was obtained from the Institutional Ethics Committee (RUHS-CMS/Ethics Comm./2022/70). Each participant provided informed consent to participate in the study.
Conflicts of interest
There are no conflicts of interest.
Author contributions
SS analyzed and interpreted the data regarding subjects. NS also interpreted the data and was a significant contributor to the manuscript. SK drafted the work or substantively revised it. All authors read and approved the final manuscript.
Article Type: Research |
Subject:
Physiology
References
1. National Institute of Mental Health. Depression. 2024. [View at Publisher]
2. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990- 2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545-602. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007;370(9590):851-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. World Health Organization. Depression and other common mental disorders: global health estimates. 2017. [View at Publisher] [Google Scholar]
5. Carney RM, Freedland KE, Stein PK, Skala JA, Hoffman P, Jaffe AS. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom Med. 2000;62(5):639-47. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Chang H-A, Chang C-C, Chen C-L, Kuo TBJ, Lu R-B, Huang S-Y. Major depression is associated with cardiac autonomic dysregulation. Acta Neuropsychiatrica. 2012;24(6):318-27. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Hartmann R, Schmidt FM, Sander C, Hegerl U. Heart Rate Variability as Indicator of Clinical State in Depression. Front Psychiatry. 2019;9:735. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Liang CS, Lee JF, Chen CC, Chang YC. Reactive heart rate variability in male patients with first-episode major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:52-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Lee CL, Chang WD. The effect of cigarette smoking on aerobic and anaerobic capacity and heart rate variability among female university students. Int J Womens Health. 2013;5:667-79. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Tarvainen MP, Niskanen J. HRV Users Guide Ver 21 Biosignal Analysis and Medical Imaging Group (BSAMIG), Department of Applied Physics, University of Eastern Finland, Kuopio. 2012:8-12. [View at Publisher] [Google Scholar]
11. Sayar K, Güleç H, Gökçe M, Ismail AK. Heart rate variability in depressed patients. Bull Clin Psychopharmacol. 2002;12(3):130-3. [View at Publisher] [Google Scholar]
12. Licht CM, de Geus EJ, Zitman FG, Hoogendijk WJ, van Dyck R, Penninx BW. Association between major depressive disorder and heart rate variability in the Netherlands study of depression and anxiety (NSEDA). Arch Gen Psychiatry. 2008;65(12):1358-67. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. World Health Organization. The ICD-10 classification of mental and behavioural disorders: Diagnostic criteria for research. Geneva: WHO,1993. [View at Publisher] [Google Scholar]
14. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Majumder S. Socioeconomic status scales: Revised Kuppuswamy, BG Prasad, and Udai Pareekh's scale updated for 2021. J Family Med Prim Care. 2021;10(11):3964-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-98. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. . Circulation. 1996;93(5):1043-65. [View at Publisher] [DOI] [Google Scholar]
18. Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141(2):122-31. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67(11):1067-74. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Singla S, Jhamb S, Singh KD, Kumar A. Depression affects autonomic system of the body? Yes, it does! J Educ Health Promot. 2020;9:217. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Bassett D. A literature review of heart rate variability in depressive and bipolar disorders. Aust N Z J Psychiatry. 2016;50(6):511-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Brunoni AR, Kemp AH, Dantas EM, Goulart AC, Nunes MA, Boggio PS, et al. Heart rate variability is a trait marker of major depressive disorder: evidence from the sertraline vs. electric current therapy to treat depression clinical study. Int J Neuropsychopharmacol. 2013 ;16(9):1937-49. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029-40. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Trivedi MH, Greer TL. Cognitive dysfunction in unipolar depression: implications for treatment. J Affect Disord. 2014;152-154:19-27. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med. 2005;67(1):S29-33. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Hong S, Park DH, Ryu SH, Ha JH, Jeon HJ. Association between Heart Rate Variability Indices and Depressed Mood in Patients with Panic Disorder. Clin Psychopharmacol Neurosci. 2022;20(4):737-746. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34(4):468-83. [View at Publisher] [PMID] [Google Scholar]
28. Porges SW. The polyvagal perspective. Biol Psychol. 2007;74(2):116-43. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






