Volume 8, Issue 2 (Journal of Clinical and Basic Research (JCBR) 2024)
jcbr 2024, 8(2): 28-33 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bola Odegbemi O, Folaranmi Olaniyan M, Abidemi Muhibi M. Impact of tenofovir, lamivudine, and dolutegravir on liver function in HIV-positive individuals in Southern Nigeria. jcbr 2024; 8 (2) :28-33
URL: http://jcbr.goums.ac.ir/article-1-453-en.html
URL: http://jcbr.goums.ac.ir/article-1-453-en.html
1- Medical Laboratory Science Department, Edo State University, Uzairue, Edo State, Nigeria ; Medical Laboratory Science Department, Nigerian Navy Hospital, Warri, Delta State, Nigeria , odegbemi21.odekunle@edouniversity.edu.ng
2- Medical Laboratory Science Department, Edo State University, Uzairue, Edo State, Nigeria
2- Medical Laboratory Science Department, Edo State University, Uzairue, Edo State, Nigeria
Full-Text [PDF 608 kb]
(885 Downloads)
| Abstract (HTML) (3438 Views)
Full-Text: (1817 Views)
Introduction
The advent of antiretroviral therapy (ART) has revolutionized HIV management, significantly improving life expectancy and quality of life for affected individuals. Despite these advancements, certain ART medications, including reverse transcriptase inhibitors (RTIs) and integrase strand transfer inhibitors (INSTIs), may induce adverse effects such as hepatic and renal toxicity (1). In Nigeria, the recent implementation of tenofovir/lamivudine/dolutegravir (TLD) regimen marks a crucial step forward in the nation's HIV/AIDS response, offering enhanced treatment efficacy, fewer side effects, and more streamlined regimens for patients (2,3).
Hepatotoxicity is a major concern in HIV treatment, especially with antiretroviral therapy (ART). While RTIs and INSTIs are crucial for suppressing viral replication and improving patient outcomes, they carry inherent risks. First-generation non-nucleoside RTIs have been associated with liver toxicity, whereas second-generation counterparts are generally considered safer (3). Understanding these risks is essential for effective treatment. Given the need to enhance HIV treatment protocols in Nigeria, investigating the hepatotoxicity linked to RTIs and INSTIs is crucial for improving patient care (4,5).
Assessing liver function is vital for HIV patients on ART due to the potential for drug-induced liver damage exacerbated by viral hepatitis coinfections and HIV-related liver inflammation. Older ART classes like NNRTIs and PIs have varying degrees of hepatotoxicity, necessitating careful monitoring of liver enzymes. Newer drugs, such as integrase inhibitors, generally offer improved liver safety profiles. Regular liver function tests are critical for early detection of liver injury and informed treatment decisions, highlighting the ongoing necessity for optimized ART regimens that balance effectiveness with minimal liver toxicity in managing HIV (3).
This study aimed to elucidate the intricate patterns of liver injury linked to TLD administration among HIV clients on TLD. This can provide critical insights to inform clinical decision-making, guide patient monitoring practices, and improve HIV management in resource-constrained environments. Specifically, this research investigated the frequency, intensity, and predisposing factors of hepatotoxicity among HIV-positive individuals receiving TLD treatment at a military healthcare facility in southern Nigeria.
Methods
A cross-sectional study was conducted on 120 HIV-positive individuals on tenofovir/lamivudine, dolutegravir (TLD), and 50 HIV-negative controls at the Nigerian Navy Hospital (NNH) in Warri. The control group was matched to the HIV-positive group in terms of sex, age, socioeconomic status, and ethnicity to minimize potential confounders and ensure the validity of the results.
Study population
The study included consenting Nigerian males and females aged 18 to 65 attending NNH Warri for healthcare services. Participants with primary chronic conditions such as liver cirrhosis, chronic obstructive pulmonary disease, cancer, and alcoholism were excluded. Simple random sampling was employed to achieve a diverse demographic representation.
Sample size calculation
The sample size for the study was determined using the following formula (6):

Hence, not less than 111 HIV-infected subjects on TLD were to be involved in this research. Nonetheless, a combined sum of 170 persons were involved in the study. A total of 120 HIV seropositive subjects and 50 HIV seronegative controls matched for age, sex, and ethnicity were recruited.
Sample collection and processing
Blood samples were collected using aseptic venipuncture, transferred into Lithium Heparin specimen bottles, and centrifuged at 4,000 RPM for 3-5 minutes to separate plasma. We stored the plasma at -20°C until biochemical analysis.
Biochemical analysis
Plasma levels of liver function parameters, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutathione S-transferases (GSTs), total protein (TP), and albumin (Alb), were measured using appropriate biochemical methods. We included control samples and commercial standards in each batch to ensure the accuracy and validity of assays.
Data analysis
The data was entered into SPSS Software version 23 for further statistical analysis. Univariate analysis included data presentation in frequency tables and charts. Statistical analysis was conducted, and the data were depicted as mean ± standard deviation. Differences in means between groups were assessed using the Student's t-test. ANOVA was used for further analysis to find the association and effects of TLD therapy on biochemical parameters. Results were regarded as significant at p < 0.05.
Results
Table 1 presents the sociodemographic characteristics of the study population, aged 18 to 65 years (Mean age: 38.6 ± 11.2 years). Among the 170 participants, 94 (55.3%) were female, and 68 (40.0%) were HIV-positive.
Table 2 outlines the characteristics of HIV-positive individuals (Mean age: 38.4 ± 11.1 years), with 68 (56.7%) females and 57 (47.5%) married participants.
Table 3 presents the data on HIV-negative individuals (Mean age: 39.0 ± 11.3 years), with 26 (52.0%) females and 23 (46.0%) married participants.
Table 4 shows the independent sample t-tests comparing liver function markers between the two groups. There were significant differences in AST, TP, Alb, and GST mean levels. HIV-positive subjects exhibited lower AST and Alb levels but higher TP and GST levels compared to HIV-negative subjects.
Table 5 presents correlation coefficients between various variables, including age, gender, ART initiation, and liver function markers. Notably, age showed a weak negative correlation with gender, and ART initiation had a moderate negative correlation with age. AST and ALT exhibited positive correlations, while negative correlations were observed between AST, ALT, TP, and Alb.
Table 6 shows the relationships between liver function parameters among HIV-negative individuals. Moderate positive correlations were observed between AST and ALT, as well as between ALT and GSTs. A strong positive correlation was found between TP and Alb.
Discussion
The significant differences in AST, TP, Alb, and GST levels between HIV-positive individuals on TLD and HIV-negative subjects suggest that TLD treatment may impact liver function parameters (7,8). Understanding these differences is crucial for healthcare providers managing HIV patients, as liver function plays a vital role in overall health and treatment outcomes. The lower AST levels among HIV-positive individuals receiving TLD suggest a potential protective effect of this antiretroviral therapy on hepatic integrity (9). However, further investigations are necessary to elucidate this observation's underlying mechanisms and clinical implications. The lack of significant difference in ALT levels between the two groups indicates that TLD treatment may not significantly affect ALT activity in HIV-positive individuals (10). Nevertheless, continuous monitoring of ALT levels remains essential to detect potential hepatotoxic effects associated with TLD therapy.
The significant differences in TP and Alb levels suggest alterations in hepatic protein synthesis among HIV-positive individuals on TLD (11,12). Elevated TP levels imply modifications in hepatic protein metabolism secondary to antiretroviral therapy, necessitating further exploration of the specific proteins involved and their clinical implications. Lower Alb levels in HIV-positive subjects on TLD may indicate hepatocellular dysfunction or impaired albumin production related to antiretroviral therapy (13).
The significant difference in GST levels suggests alterations in hepatic detoxification pathways among HIV-positive individuals (14). Elevated GST levels, particularly in those on TLD, indicate increased hepatic oxidative stress and cellular injury, emphasizing the need to evaluate hepatic antioxidant capacity and detoxification mechanisms in HIV patients on TLD.
The observed correlations between age, gender, and liver enzymes highlight the complex interplay between these factors, HIV, and liver function. While age exhibited a modest association with liver enzymes, gender showed minimal correlation, which underscores the need for further research to fully understand these relationships.
The strong positive correlation between ALT and GSTs suggests a robust association between liver enzyme elevation and oxidative stress, warranting additional investigation into potential liver damage. The findings contribute to our understanding of liver function in HIV/AIDS patients, guiding clinical management and future research endeavors.
Our findings revealed significant differences in AST, TP, Alb, and GST levels between HIV-positive individuals on TLD and HIV-negative subjects. These observed variations in liver function markers suggest a notable impact of TLD therapy on hepatic parameters among HIV-positive patients (7,8). Understanding these differences is crucial for healthcare providers managing HIV patients, as liver function plays a pivotal role in overall health and treatment outcomes.
Aspartate aminotransferase levels exhibited a considerable decrease in individuals with HIV receiving TLD compared to HIV-negative subjects, implying a potential protective effect of this antiretroviral regimen on hepatic integrity (9). However, further investigations are warranted to elucidate this observation's underlying mechanisms and clinical implications. In contrast, alanine aminotransferase levels remained comparable between the two groups, suggesting that TLD treatment may not significantly alter ALT activity in HIV-positive individuals (10). Nevertheless, continuous monitoring of ALT levels remains essential to detect potential hepatotoxic effects associated with TLD therapy.
The significant difference in TP levels suggests alterations in hepatic protein synthesis among HIV-positive individuals on TLD (11). Elevated TP levels imply modifications in hepatic protein metabolism secondary to antiretroviral therapy, necessitating further exploration of the specific proteins involved and their clinical implications. Similarly, the significant difference in plasma Alb levels highlights the impact of TLD treatment on hepatic albumin synthesis. Lower Alb levels in HIV-positive subjects on TLD suggest potential hepatocellular dysfunction or impaired albumin production related to antiretroviral therapy (12). These findings, akin to those reported by Abba et al. in Cameroon, underscore the importance of comprehensive assessment and monitoring of hepatic synthetic function in HIV patients on TLD (13).
The significant difference in GST levels suggests alterations in hepatic detoxification pathways among HIV-positive individuals (14). Elevated GST levels, particularly in those on TLD, indicate increased hepatic oxidative stress and cellular injury, emphasizing the need to evaluate hepatic antioxidant capacity and detoxification mechanisms in HIV patients on TLD.
The independent sample t-tests comparing liver function markers between the groups revealed significant differences in AST, TP, Alb, and GST levels. The lower mean values of AST and Alb in HIV-positive subjects suggest potential liver dysfunction compared to HIV-negative individuals. Conversely, higher mean values of TP and GSTs in HIV-positive subjects indicate possible liver stress or inflammation due to HIV infection.
These findings align with previous studies that have reported altered liver function in HIV-positive individuals. For instance, Dusingize et al. found significantly higher levels of liver enzymes, such as AST and ALT, in HIV-positive patients compared to HIV-negative controls, suggesting liver dysfunction in HIV-positive individuals (15). However, Osakunor et al. in Ghana reported no significant change in liver enzymes among antiretroviral therapy (ART) clients, with a caveat that liver enzyme monitoring should be ensured among HIV clients despite the findings (16).
Age exhibited a modest association with liver enzymes, while gender showed minimal correlation, emphasizing the need for further clinical research to understand these relationships fully. The strong positive correlation between ALT and GSTs suggests a robust association between liver enzyme elevation and oxidative stress, warranting additional investigation into potential liver damage.
The correlation analysis provides insights into the relationships between variables, contextualizing the results and identifying consistencies across different populations. The findings contribute to our understanding of liver function in HIV/AIDS patients, guiding clinical management and future research endeavors.
The negative and weak correlation observed between age and gender in this study is consistent with prior findings (17,18), indicating that the relationship between age and gender may not be substantial within HIV/AIDS cohorts. Similarly, the negative, moderate correlation between the duration of ART intake and the age of starting ART is consistent with findings from other studies (19,20), indicating that older individuals may initiate ART later in their disease course.
The positive correlation observed between AST and ALT levels is a common finding in studies assessing liver function in HIV/AIDS patients (21-23). This suggests that elevations in one liver enzyme may be accompanied by elevations in the other, reflecting potential liver injury or inflammation. However, Wood et al. (2021) reported that long-term use of integrase strand transfer inhibitor (INSTI)-based ART reduces liver enzyme elevation by half compared to non-nucleoside reverse transcriptase inhibitors (NNRTI) (24). This observed difference may, however, be due to race, ethnicity, and study design variations.
The weak positive correlation between age and AST (0.137) suggests a slight tendency for older patients to have higher AST levels. This finding is inconclusive and requires further investigation with a larger sample size. Interestingly, a weak negative correlation between age and ALT (-0.066) was observed, contradicting what was reported by other studies (25). No significant correlations were found between gender and either AST or ALT levels. This aligns with some studies suggesting minimal influence of gender on these enzymes in healthy individuals (26).
The negative correlations between AST, ALT, and other markers of liver function, such as TP and Alb, are consistent with existing literature (27,28), highlighting the impact of liver dysfunction on these biochemical parameters. Regarding the correlations involving age and gender with AST and ALT levels, the findings may vary across studies. While some studies may report similar trends (15), others may find conflicting results (29), emphasizing the need for further investigation into the influence of age and gender on liver enzyme levels in HIV/AIDS patients.
Accordingly, the correlations identified in this study contribute to our understanding of liver function in HIV/AIDS patients receiving ART. We can discern patterns and discrepancies by comparing these results with similar studies, ultimately guiding clinical management and future research endeavors. The moderate positive correlation between AST and ALT in Table 6 aligns with established knowledge, suggesting similar mechanisms for liver enzyme elevation in HIV-positive individuals (21).
The strong positive correlation (0.634) observed between ALT and GSTs among HIV seronegative subjects is an intriguing finding. While not directly explored in the context of HIV, increased GST levels can be a compensatory response to oxidative stress, which can occur in liver damage (30). Further research is needed to understand this association better. The strong positive correlation (0.654) between TP and Alb is consistent with expectations, as albumin is the major protein produced by the liver (31).
Both groups showed positive correlations between AST and ALT, suggesting similar underlying mechanisms for liver enzyme elevation. However, the study design cannot definitively determine if HIV or ART medication contributes to the observed correlations in the HIV-positive group. This study underscores the importance of monitoring liver function in HIV-positive individuals and addressing potential complications associated with HIV and antiretroviral therapy. Continued research is essential to elucidate the mechanisms underlying liver dysfunction and develop targeted interventions to improve liver health in this population.
Conclusion
Our study reveals significant differences in AST, TP, Alb, and GST levels among HIV-positive individuals, suggesting TLD treatment's impact on liver function. While TLD seems to lower AST levels, implying liver protection (32), further research is needed to understand its mechanisms. Conversely, ALT levels showed no significant change, indicating minimal impact on liver activity. Continuous monitoring remains crucial to detect potential hepatotoxic effects of TLD therapy.
The significant differences in TP and Alb levels underscore alterations in hepatic protein synthesis among HIV seropositive individuals on TLD (33). Elevated TP levels suggest modifications in hepatic protein metabolism secondary to antiretroviral therapy, while lower Alb levels may indicate hepatocellular dysfunction (11). Similar findings reported in Cameroon emphasize the importance of comprehensive assessment and monitoring of hepatic synthetic function in HIV patients on TLD (13). Moreover, significant differences in GST levels highlight alterations in hepatic detoxification pathways (34), necessitating the evaluation of antioxidant capacity and detoxification mechanisms in HIV patients receiving TLD.
Comparing our findings with previous research suggests consistency in associations between age, gender, and liver enzymes (17,18). The observed positive correlation between AST and
ALT levels align with existing literature on liver function in HIV/AIDS patients (22,23), indicating potential liver injury or inflammation. However, differences in enzyme elevation between INSTI-based and NNRTI-based ART regimens warrant further investigation (35). The negative correlations between AST, ALT, and other liver markers, along with the varying associations of age and gender, contribute to our understanding of liver function in this population (27,28).
Reverse transcriptase inhibitors and INSTI therapy benefit HIV clients, but metabolic syndrome and associated liver disease pose growing risks as HIV-positive individuals age (36). The correlations in this study offer insights into the intricate relationship between HIV, ART, and liver function, guiding clinical care and future research. However, our study had limitations, including a small sample size and a lack of exploration of potential confounding factors, such as other viral infections among participants, which may have impacted our findings. Longitudinal studies are needed to uncover mechanisms behind liver dysfunction in HIV patients and develop targeted interventions for hepatic health improvement.
Acknowledgement
We appreciate the Nigerian Ministry of Defense Health Implementation Program and the Nigerian Navy Hospital Warri management for providing a supportive environment and approving this study.
Funding sources
There was no external funding for this research
Ethical statement
We secured ethical approval from the appropriate review board and obtained relevant administrative approval before the commencement of the study. Informed consent was obtained from all participants that met the inclusion criteria. Data collection involved administering a semi-structured questionnaire to gather clinical, demographic, and behavioral information.
Conflicts of interest
The authors declare no conflicts of interest.
Author contributions
OBO contributed to research design, sample analysis, data analysis, and manuscript writing.
MFO contributed to research design and overall research supervision,
MAM contributed to the research design, sample selection, and data analysis.
The advent of antiretroviral therapy (ART) has revolutionized HIV management, significantly improving life expectancy and quality of life for affected individuals. Despite these advancements, certain ART medications, including reverse transcriptase inhibitors (RTIs) and integrase strand transfer inhibitors (INSTIs), may induce adverse effects such as hepatic and renal toxicity (1). In Nigeria, the recent implementation of tenofovir/lamivudine/dolutegravir (TLD) regimen marks a crucial step forward in the nation's HIV/AIDS response, offering enhanced treatment efficacy, fewer side effects, and more streamlined regimens for patients (2,3).
Hepatotoxicity is a major concern in HIV treatment, especially with antiretroviral therapy (ART). While RTIs and INSTIs are crucial for suppressing viral replication and improving patient outcomes, they carry inherent risks. First-generation non-nucleoside RTIs have been associated with liver toxicity, whereas second-generation counterparts are generally considered safer (3). Understanding these risks is essential for effective treatment. Given the need to enhance HIV treatment protocols in Nigeria, investigating the hepatotoxicity linked to RTIs and INSTIs is crucial for improving patient care (4,5).
Assessing liver function is vital for HIV patients on ART due to the potential for drug-induced liver damage exacerbated by viral hepatitis coinfections and HIV-related liver inflammation. Older ART classes like NNRTIs and PIs have varying degrees of hepatotoxicity, necessitating careful monitoring of liver enzymes. Newer drugs, such as integrase inhibitors, generally offer improved liver safety profiles. Regular liver function tests are critical for early detection of liver injury and informed treatment decisions, highlighting the ongoing necessity for optimized ART regimens that balance effectiveness with minimal liver toxicity in managing HIV (3).
This study aimed to elucidate the intricate patterns of liver injury linked to TLD administration among HIV clients on TLD. This can provide critical insights to inform clinical decision-making, guide patient monitoring practices, and improve HIV management in resource-constrained environments. Specifically, this research investigated the frequency, intensity, and predisposing factors of hepatotoxicity among HIV-positive individuals receiving TLD treatment at a military healthcare facility in southern Nigeria.
Methods
A cross-sectional study was conducted on 120 HIV-positive individuals on tenofovir/lamivudine, dolutegravir (TLD), and 50 HIV-negative controls at the Nigerian Navy Hospital (NNH) in Warri. The control group was matched to the HIV-positive group in terms of sex, age, socioeconomic status, and ethnicity to minimize potential confounders and ensure the validity of the results.
Study population
The study included consenting Nigerian males and females aged 18 to 65 attending NNH Warri for healthcare services. Participants with primary chronic conditions such as liver cirrhosis, chronic obstructive pulmonary disease, cancer, and alcoholism were excluded. Simple random sampling was employed to achieve a diverse demographic representation.
Sample size calculation
The sample size for the study was determined using the following formula (6):

Hence, not less than 111 HIV-infected subjects on TLD were to be involved in this research. Nonetheless, a combined sum of 170 persons were involved in the study. A total of 120 HIV seropositive subjects and 50 HIV seronegative controls matched for age, sex, and ethnicity were recruited.
Sample collection and processing
Blood samples were collected using aseptic venipuncture, transferred into Lithium Heparin specimen bottles, and centrifuged at 4,000 RPM for 3-5 minutes to separate plasma. We stored the plasma at -20°C until biochemical analysis.
Biochemical analysis
Plasma levels of liver function parameters, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutathione S-transferases (GSTs), total protein (TP), and albumin (Alb), were measured using appropriate biochemical methods. We included control samples and commercial standards in each batch to ensure the accuracy and validity of assays.
Data analysis
The data was entered into SPSS Software version 23 for further statistical analysis. Univariate analysis included data presentation in frequency tables and charts. Statistical analysis was conducted, and the data were depicted as mean ± standard deviation. Differences in means between groups were assessed using the Student's t-test. ANOVA was used for further analysis to find the association and effects of TLD therapy on biochemical parameters. Results were regarded as significant at p < 0.05.
Results
Table 1 presents the sociodemographic characteristics of the study population, aged 18 to 65 years (Mean age: 38.6 ± 11.2 years). Among the 170 participants, 94 (55.3%) were female, and 68 (40.0%) were HIV-positive.
Table 3 presents the data on HIV-negative individuals (Mean age: 39.0 ± 11.3 years), with 26 (52.0%) females and 23 (46.0%) married participants.
Table 4 shows the independent sample t-tests comparing liver function markers between the two groups. There were significant differences in AST, TP, Alb, and GST mean levels. HIV-positive subjects exhibited lower AST and Alb levels but higher TP and GST levels compared to HIV-negative subjects.
Table 5 presents correlation coefficients between various variables, including age, gender, ART initiation, and liver function markers. Notably, age showed a weak negative correlation with gender, and ART initiation had a moderate negative correlation with age. AST and ALT exhibited positive correlations, while negative correlations were observed between AST, ALT, TP, and Alb.
Table 6 shows the relationships between liver function parameters among HIV-negative individuals. Moderate positive correlations were observed between AST and ALT, as well as between ALT and GSTs. A strong positive correlation was found between TP and Alb.
|
Table 2. Demographic characteristics of HIV seropositive subjects at Nigerian Navy Hospital Warri
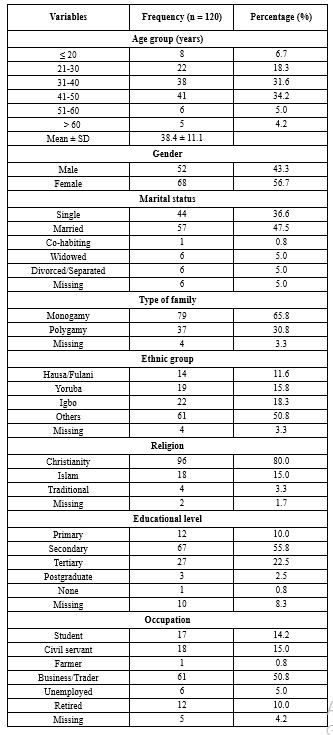 |
Table 3. Demographic characteristics of HIV seronegative subjects attending Nigerian Navy Hospital Warri Table 4. Effect of TLD on liver function parameters among HIV seropositive participants at NNH Warri 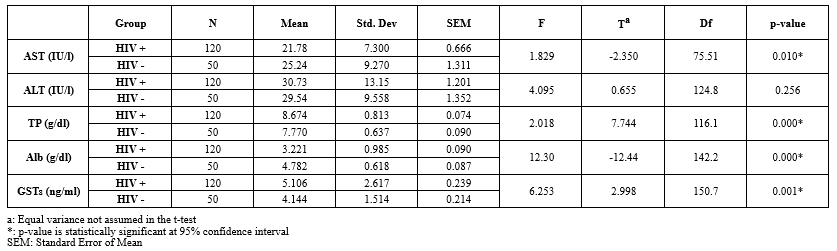 |
Table 5. Pearson correlations between sociodemographic characteristics years of therapy and liver function variables among HIV seropositive subjects attending NNH Warri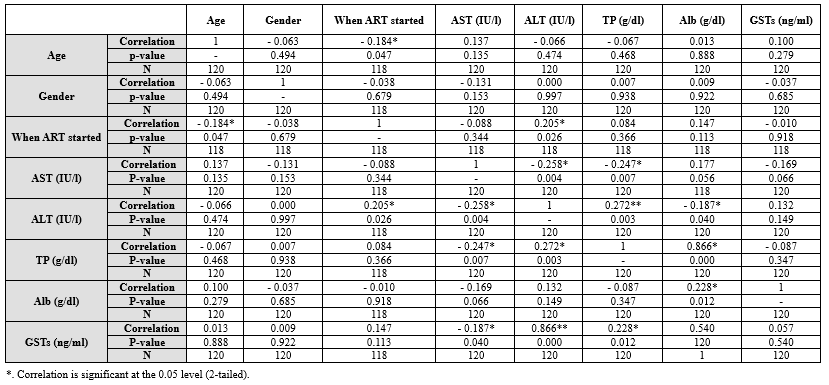 Table 6. Pearson correlations between liver function variables among HIV seronegative subjects attending NNH Warri 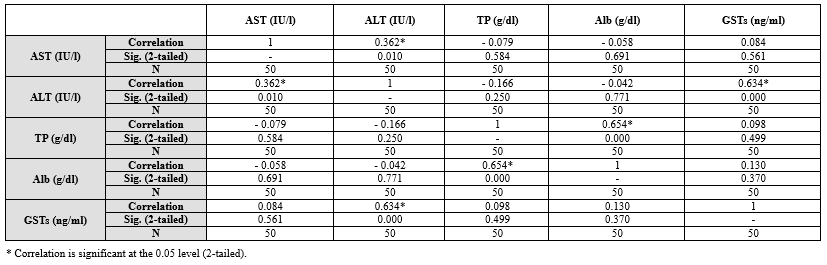 |
Discussion
The significant differences in AST, TP, Alb, and GST levels between HIV-positive individuals on TLD and HIV-negative subjects suggest that TLD treatment may impact liver function parameters (7,8). Understanding these differences is crucial for healthcare providers managing HIV patients, as liver function plays a vital role in overall health and treatment outcomes. The lower AST levels among HIV-positive individuals receiving TLD suggest a potential protective effect of this antiretroviral therapy on hepatic integrity (9). However, further investigations are necessary to elucidate this observation's underlying mechanisms and clinical implications. The lack of significant difference in ALT levels between the two groups indicates that TLD treatment may not significantly affect ALT activity in HIV-positive individuals (10). Nevertheless, continuous monitoring of ALT levels remains essential to detect potential hepatotoxic effects associated with TLD therapy.
The significant differences in TP and Alb levels suggest alterations in hepatic protein synthesis among HIV-positive individuals on TLD (11,12). Elevated TP levels imply modifications in hepatic protein metabolism secondary to antiretroviral therapy, necessitating further exploration of the specific proteins involved and their clinical implications. Lower Alb levels in HIV-positive subjects on TLD may indicate hepatocellular dysfunction or impaired albumin production related to antiretroviral therapy (13).
The significant difference in GST levels suggests alterations in hepatic detoxification pathways among HIV-positive individuals (14). Elevated GST levels, particularly in those on TLD, indicate increased hepatic oxidative stress and cellular injury, emphasizing the need to evaluate hepatic antioxidant capacity and detoxification mechanisms in HIV patients on TLD.
The observed correlations between age, gender, and liver enzymes highlight the complex interplay between these factors, HIV, and liver function. While age exhibited a modest association with liver enzymes, gender showed minimal correlation, which underscores the need for further research to fully understand these relationships.
The strong positive correlation between ALT and GSTs suggests a robust association between liver enzyme elevation and oxidative stress, warranting additional investigation into potential liver damage. The findings contribute to our understanding of liver function in HIV/AIDS patients, guiding clinical management and future research endeavors.
Our findings revealed significant differences in AST, TP, Alb, and GST levels between HIV-positive individuals on TLD and HIV-negative subjects. These observed variations in liver function markers suggest a notable impact of TLD therapy on hepatic parameters among HIV-positive patients (7,8). Understanding these differences is crucial for healthcare providers managing HIV patients, as liver function plays a pivotal role in overall health and treatment outcomes.
Aspartate aminotransferase levels exhibited a considerable decrease in individuals with HIV receiving TLD compared to HIV-negative subjects, implying a potential protective effect of this antiretroviral regimen on hepatic integrity (9). However, further investigations are warranted to elucidate this observation's underlying mechanisms and clinical implications. In contrast, alanine aminotransferase levels remained comparable between the two groups, suggesting that TLD treatment may not significantly alter ALT activity in HIV-positive individuals (10). Nevertheless, continuous monitoring of ALT levels remains essential to detect potential hepatotoxic effects associated with TLD therapy.
The significant difference in TP levels suggests alterations in hepatic protein synthesis among HIV-positive individuals on TLD (11). Elevated TP levels imply modifications in hepatic protein metabolism secondary to antiretroviral therapy, necessitating further exploration of the specific proteins involved and their clinical implications. Similarly, the significant difference in plasma Alb levels highlights the impact of TLD treatment on hepatic albumin synthesis. Lower Alb levels in HIV-positive subjects on TLD suggest potential hepatocellular dysfunction or impaired albumin production related to antiretroviral therapy (12). These findings, akin to those reported by Abba et al. in Cameroon, underscore the importance of comprehensive assessment and monitoring of hepatic synthetic function in HIV patients on TLD (13).
The significant difference in GST levels suggests alterations in hepatic detoxification pathways among HIV-positive individuals (14). Elevated GST levels, particularly in those on TLD, indicate increased hepatic oxidative stress and cellular injury, emphasizing the need to evaluate hepatic antioxidant capacity and detoxification mechanisms in HIV patients on TLD.
The independent sample t-tests comparing liver function markers between the groups revealed significant differences in AST, TP, Alb, and GST levels. The lower mean values of AST and Alb in HIV-positive subjects suggest potential liver dysfunction compared to HIV-negative individuals. Conversely, higher mean values of TP and GSTs in HIV-positive subjects indicate possible liver stress or inflammation due to HIV infection.
These findings align with previous studies that have reported altered liver function in HIV-positive individuals. For instance, Dusingize et al. found significantly higher levels of liver enzymes, such as AST and ALT, in HIV-positive patients compared to HIV-negative controls, suggesting liver dysfunction in HIV-positive individuals (15). However, Osakunor et al. in Ghana reported no significant change in liver enzymes among antiretroviral therapy (ART) clients, with a caveat that liver enzyme monitoring should be ensured among HIV clients despite the findings (16).
Age exhibited a modest association with liver enzymes, while gender showed minimal correlation, emphasizing the need for further clinical research to understand these relationships fully. The strong positive correlation between ALT and GSTs suggests a robust association between liver enzyme elevation and oxidative stress, warranting additional investigation into potential liver damage.
The correlation analysis provides insights into the relationships between variables, contextualizing the results and identifying consistencies across different populations. The findings contribute to our understanding of liver function in HIV/AIDS patients, guiding clinical management and future research endeavors.
The negative and weak correlation observed between age and gender in this study is consistent with prior findings (17,18), indicating that the relationship between age and gender may not be substantial within HIV/AIDS cohorts. Similarly, the negative, moderate correlation between the duration of ART intake and the age of starting ART is consistent with findings from other studies (19,20), indicating that older individuals may initiate ART later in their disease course.
The positive correlation observed between AST and ALT levels is a common finding in studies assessing liver function in HIV/AIDS patients (21-23). This suggests that elevations in one liver enzyme may be accompanied by elevations in the other, reflecting potential liver injury or inflammation. However, Wood et al. (2021) reported that long-term use of integrase strand transfer inhibitor (INSTI)-based ART reduces liver enzyme elevation by half compared to non-nucleoside reverse transcriptase inhibitors (NNRTI) (24). This observed difference may, however, be due to race, ethnicity, and study design variations.
The weak positive correlation between age and AST (0.137) suggests a slight tendency for older patients to have higher AST levels. This finding is inconclusive and requires further investigation with a larger sample size. Interestingly, a weak negative correlation between age and ALT (-0.066) was observed, contradicting what was reported by other studies (25). No significant correlations were found between gender and either AST or ALT levels. This aligns with some studies suggesting minimal influence of gender on these enzymes in healthy individuals (26).
The negative correlations between AST, ALT, and other markers of liver function, such as TP and Alb, are consistent with existing literature (27,28), highlighting the impact of liver dysfunction on these biochemical parameters. Regarding the correlations involving age and gender with AST and ALT levels, the findings may vary across studies. While some studies may report similar trends (15), others may find conflicting results (29), emphasizing the need for further investigation into the influence of age and gender on liver enzyme levels in HIV/AIDS patients.
Accordingly, the correlations identified in this study contribute to our understanding of liver function in HIV/AIDS patients receiving ART. We can discern patterns and discrepancies by comparing these results with similar studies, ultimately guiding clinical management and future research endeavors. The moderate positive correlation between AST and ALT in Table 6 aligns with established knowledge, suggesting similar mechanisms for liver enzyme elevation in HIV-positive individuals (21).
The strong positive correlation (0.634) observed between ALT and GSTs among HIV seronegative subjects is an intriguing finding. While not directly explored in the context of HIV, increased GST levels can be a compensatory response to oxidative stress, which can occur in liver damage (30). Further research is needed to understand this association better. The strong positive correlation (0.654) between TP and Alb is consistent with expectations, as albumin is the major protein produced by the liver (31).
Both groups showed positive correlations between AST and ALT, suggesting similar underlying mechanisms for liver enzyme elevation. However, the study design cannot definitively determine if HIV or ART medication contributes to the observed correlations in the HIV-positive group. This study underscores the importance of monitoring liver function in HIV-positive individuals and addressing potential complications associated with HIV and antiretroviral therapy. Continued research is essential to elucidate the mechanisms underlying liver dysfunction and develop targeted interventions to improve liver health in this population.
Conclusion
Our study reveals significant differences in AST, TP, Alb, and GST levels among HIV-positive individuals, suggesting TLD treatment's impact on liver function. While TLD seems to lower AST levels, implying liver protection (32), further research is needed to understand its mechanisms. Conversely, ALT levels showed no significant change, indicating minimal impact on liver activity. Continuous monitoring remains crucial to detect potential hepatotoxic effects of TLD therapy.
The significant differences in TP and Alb levels underscore alterations in hepatic protein synthesis among HIV seropositive individuals on TLD (33). Elevated TP levels suggest modifications in hepatic protein metabolism secondary to antiretroviral therapy, while lower Alb levels may indicate hepatocellular dysfunction (11). Similar findings reported in Cameroon emphasize the importance of comprehensive assessment and monitoring of hepatic synthetic function in HIV patients on TLD (13). Moreover, significant differences in GST levels highlight alterations in hepatic detoxification pathways (34), necessitating the evaluation of antioxidant capacity and detoxification mechanisms in HIV patients receiving TLD.
Comparing our findings with previous research suggests consistency in associations between age, gender, and liver enzymes (17,18). The observed positive correlation between AST and
ALT levels align with existing literature on liver function in HIV/AIDS patients (22,23), indicating potential liver injury or inflammation. However, differences in enzyme elevation between INSTI-based and NNRTI-based ART regimens warrant further investigation (35). The negative correlations between AST, ALT, and other liver markers, along with the varying associations of age and gender, contribute to our understanding of liver function in this population (27,28).
Reverse transcriptase inhibitors and INSTI therapy benefit HIV clients, but metabolic syndrome and associated liver disease pose growing risks as HIV-positive individuals age (36). The correlations in this study offer insights into the intricate relationship between HIV, ART, and liver function, guiding clinical care and future research. However, our study had limitations, including a small sample size and a lack of exploration of potential confounding factors, such as other viral infections among participants, which may have impacted our findings. Longitudinal studies are needed to uncover mechanisms behind liver dysfunction in HIV patients and develop targeted interventions for hepatic health improvement.
Acknowledgement
We appreciate the Nigerian Ministry of Defense Health Implementation Program and the Nigerian Navy Hospital Warri management for providing a supportive environment and approving this study.
Funding sources
There was no external funding for this research
Ethical statement
We secured ethical approval from the appropriate review board and obtained relevant administrative approval before the commencement of the study. Informed consent was obtained from all participants that met the inclusion criteria. Data collection involved administering a semi-structured questionnaire to gather clinical, demographic, and behavioral information.
Conflicts of interest
The authors declare no conflicts of interest.
Author contributions
OBO contributed to research design, sample analysis, data analysis, and manuscript writing.
MFO contributed to research design and overall research supervision,
MAM contributed to the research design, sample selection, and data analysis.
Article Type: Research |
Subject:
Biochemistry
References
1. Echefu SN, Udosen JE, Akwiwu EC, Akpotuzor JO, Obeagu EI. Effect of Dolutegravir regimen on hematological parameters, CD4 count, and viral load in people living with HIV infection in South Eastern Nigeria. Medicine (Baltimore). 2023;102(47):e35910. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. World Health Organization. HIV drug resistance report 2019. 2019. [View at Publisher] [Google Scholar]
3. Benedicto AM, Fuster-Martínez I, Tosca J, Esplugues JV, Blas-García A, Apostolova N. NNRTI and liver damage: Evidence of their association and the mechanisms involved. Cells. 2021;10(7):1687. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Awofala AA, Ogundele OE. HIV epidemiology in Nigeria. Saudi J Biol Sci. 2018;25(4):697-703. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Bassey AE, Miteu GD. A review of current trends in HIV epidemiology, surveillance, and control in Nigeria. Ann Med Surg (Lond). 2023;85(5):1790-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Lwanga SK, Lemeshow S, World Health Organization. Sample size determination in health studies: a practical manual. World Health Organization; 1991. [View at Publisher] [Google Scholar]
7. Wu L, Jin C, Bai S, Davies H, Rao H, Liang Y, et al. The effect of highly active antiretroviral therapy on liver function in HIV-infected pediatric patients with or without hepatitis virus co-infection. J Res Med Sci. 2015;20(2):127-32. [View at Publisher] [PMID] [Google Scholar]
8. Chwiki S, Campos MM, McLaughlin ME, Kleiner DE, Kovacs JA, Morse CG, et al. Adverse effects of antiretroviral therapy on liver hepatocytes and endothelium in HIV patients: An ultrastructural perspective. Ultrastruct Pathol. 2017;41(2):186-95. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Moya-Salazar J, Barrial-Vega M, Arrieta-Calderón R, Contreras-Pulache H. Changes in liver function test levels in HIV patients undergoing highly active antiretroviral therapy (HAART): Longitudinal study in Lima, Peru. Revista de la Facultad de Medicina. 2022;70(1):e86775. [View at Publisher] [DOI] [Google Scholar]
10. Odegbemi OB, Olaniyan MF, Muhibi MA. Hepatic Toxicity Assessment in HIV's Interaction with Reverse Transcriptase and Integrase Strand Transfer Inhibitors at a Military Hospital, Southsouth Nigeria. medRxiv. 2024. [View at Publisher] [DOI] [Google Scholar]
11. Shedrac AE, Haruna M, Gloria EA, Nma YB, Titus EF, Musa DA, et al. Anti-retroviral therapy and serum protein levels in HIV-1 seropositive patients: A five-year retrospective study. medRxiv. 2020. [View at Publisher] [DOI] [Google Scholar]
12. Abubakar MG, Abduljalil MM, Bola-Alaka G, Nasiru YI. Influence of ARVs on Some Biochemical Changes in Liver Non Enzymatic Markers of HIV Positive Patients Attending Specialist Hospital Sokoto, Nigeria. Nigerian Journal of Basic and Applied Science. 2015;23(1):45-50. [View at Publisher] [DOI] [Google Scholar]
13. Abba A, Fokam J, Kamgaing RS, Yimga JF, Ka’e AC, Nka AD, et al. Correlation between the immuno-virological response and the nutritional profile of treatment-experienced HIV-infected patients in the East region of Cameroon. PLoS One. 2021;16(5):e0229550. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Quaye O, Kuleape JA, Bonney EY, Puplampu P, Tagoe EA. Imbalance of antioxidant enzymes activities and trace elements levels in Ghanaian HIV-infected patients. PLoS One. 2019;14(7):e0220181. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Dusingize JC, Hoover DR, Shi Q, Mutimura E, Rudakemwa E, Ndacyayisenga V, et al. Association of Abnormal Liver Function Parameters with HIV Serostatus and CD4 Count in Antiretroviral-Naive Rwandan Women. AIDS Res Hum Retroviruses. 2015;31(7):723-30. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Osakunor DN, Obirikorang C, Fianu V, Asare I, Dakorah M. Hepatic Enzyme Alterations in HIV Patients on Antiretroviral Therapy: A Case-Control Study in a Hospital Setting in Ghana. PLoS One. 2015;10(8):e0134449. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Chory A, Gillette E, Callen G, Wachira J, Sam-Agudu NA, Bond K, et al. Gender differences in HIV knowledge among adolescents and young people in low-and middle-income countries: A systematic review. Front Reprod Health. 2023;5:1154395. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Bernard C, Font H, Diallo Z, Ahonon R, Tine JM, Abouo FN, et al. Effects of Age, Level of Education and HIV Status on Cognitive Performance in West African Older Adults: The West Africa IeDEA Cohort Collaboration. AIDS Behav. 2021;25(10):3316-26. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Aliyu MH, Blevins M, Parrish DD, Megazzini KM, Gebi UI, Muhammad MY, et al. Risk factors for delayed initiation of combination antiretroviral therapy in rural north central Nigeria. J Acquir Immune Defic Syndr. 2014;65(2):e41-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Nkalubo J, Mugaba M, Asasira I, Nakiganda R, Namutebi F, Arnaud NN, et al. Factors associated with readiness to start antiretroviral therapy (ART) among young people (15-24 years) at four HIV clinics in Mulago Hospital, Uganda. Afr Health Sci. 2021;21(4):1603-14. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Mata-Marín JA, Gaytán-Martínez J, Grados-Chavarría BH, Fuentes-Allen JL, Arroyo-Anduiza CI, Alfaro-Mejía A. Correlation between HIV viral load and aminotransferases as liver damage markers in HIV infected naive patients: A concordance cross-sectional study. Virol J. 2009;6:181. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Lapadula G, Costarelli S, Chatenoud L, Castelli F, Astuti N, Giambenedetto SD, et al. Risk of liver enzyme elevation during treatment with ritonavir-boosted protease inhibitors among HIV-monoinfected and HIV/HCV-coinfected patients. J Acquir Immune Defic Syndr. 2015;69(3):312-318. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Mataranyika PA, Kibuule D, Kalemeera F, Kaura H, Rennie TW. Liver enzyme elevations in a cohort of HIV/AIDS patients on first-line antiretroviral therapy in Namibia: Findings and implications. Alexandria Journal of Medicine. 2018;54(1):49-56. [View at Publisher] [DOI] [Google Scholar]
24. Wood S, Won SH, Hsieh HC, Lalani T, Kronmann K, Maves RC, et al. Risk Factors Associated With Chronic Liver Enzyme Elevation in Persons With HIV Without Hepatitis B or C Coinfection in the Combination Antiretroviral Therapy Era. Open Forum Infect Dis. 2021;8(3):ofab076. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Sung KC, Lee MY, Lee JY, Lee SH, Kim SH, Kim SH. Utility of ALT concentration in men and women with nonalcoholic fatty liver disease: Cohort study. J Clin Med. 2019;8(4):445. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Mennecozzi M, Landesmann B, Palosaari T, Harris G, Whelan M. Hepatotoxicity screening strategies: Comparison of liver enzyme release and NMR-based metabolomics in HepG2 cells and primary rat hepatocytes. Archives of Toxicology. 2012;86(10):1581-1592.
27. Hosen MB, Karmokar NC, Karim MF, Al Mahmud R, Uddin M. Association of AST, ALT, ALB and total protein with beta-thalassemia in Bangladeshi population. Int J Adv Res. 2015;3(1):991-5. [View at Publisher] [Google Scholar]
28. Song X, Zha Y, Liu J, He P, He L. Associations between liver function parameters and poor clinical outcomes in peritoneal dialysis patients. Ther Apher Dial. 2023;27(1):12-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Ocama P, Castelnuovo B, Kamya MR, Kirk GD, Reynolds SJ, Kiragga A, et al. Low frequency of liver enzyme elevation in HIV-infected patients attending a large urban treatment centre in Uganda. Int J STD AIDS. 2010;21(8):553-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Mazari AMA, Zhang L, Ye ZW, Zhang J, Tew KD, Townsend DM. The multifaceted role of glutathione S-transferases in health and disease. Biomolecules. 2023;13(4):688. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Belinskaia DA, Voronina PA, Shmurak VI, Jenkins RO, Goncharov NV. Serum albumin in health and disease: Esterase, antioxidant, transporting and signaling properties. Int J Mol Sci. 2021;22(19):10318. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Qin F, Jiang J, Qin C, Huang Y, Liang B, Xu Y, et al. Liver damage in patients living with HIV on antiretroviral treatment with normal baseline liver function and without HBV/HCV infection: An 11-year retrospective cohort study in Guangxi, China. BMJ Open. 2019;9(4):e023140. [View at Publisher] [DOI] [PMID] [Google Scholar]
33. Igwe CU, Ewuga EE, Ujowundu CO, Onyeocha IO, Onwuliri VA. Serum protein concentration and amino acid profile of HIV/HBV co-infected subjects on HAART in Plateau State, Nigeria. Afr Health Sci. 2022;22(1):418-30. [View at Publisher] [DOI] [PMID] [Google Scholar]
34. Quaye O, Kuleape JA, Bonney EY, Puplampu P, Tagoe EA. Imbalance of antioxidant enzymes activities and trace elements levels in Ghanaian HIV-infected patients. PLoS One. 2019;14(7):e0220181. [View at Publisher] [DOI] [PMID] [Google Scholar]
35. Biały M, Czarnecki M, Inglot M. Impact of Combination Antiretroviral Treatment on Liver Metabolic Health in HIV-Infected Persons. Viruses. 2023;15(12):2432. [View at Publisher] [DOI] [PMID] [Google Scholar]
36. Taramasso L, Lorenzini P, Di Biagio A, Lichtner M, Marchetti G, Rossotti R, et al. Incidence and risk factors for liver enzyme elevation among naive HIV-1-infected patients receiving ART in the ICONA cohort. J Antimicrob Chemother. 2019;74(11):3295-304. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







