Volume 6, Issue 2 (Journal of Clinical and Basic Research (JCBR) 2022)
jcbr 2022, 6(2): 12-20 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Boddapati A, Inuganti Venkata R, Riyaz P, B.V V, Deepak V. Hematological and Biochemical Abnormalities in Pregnancy-Induced Hypertension. jcbr 2022; 6 (2) :12-20
URL: http://jcbr.goums.ac.ir/article-1-355-en.html
URL: http://jcbr.goums.ac.ir/article-1-355-en.html
1- Department of Pathology, NRI Medical College, Mangalagiri, Guntur-522 503, Andhra Pradesh, India
2- Department of Pathology, Prathima institute of medical Sciences, Karimnagar, Telangana, India ,research.nmch@rediffmail.com
3- Department of Pathology, Naryana Medical College, Nellore, A.P, India
4- Department of Pathology, Surabhi Institute of Medical Sciences, Siddipet, Telangana, India
2- Department of Pathology, Prathima institute of medical Sciences, Karimnagar, Telangana, India ,
3- Department of Pathology, Naryana Medical College, Nellore, A.P, India
4- Department of Pathology, Surabhi Institute of Medical Sciences, Siddipet, Telangana, India
Full-Text [PDF 489 kb]
(1959 Downloads)
| Abstract (HTML) (3287 Views)
Full-Text: (1895 Views)
INTRODUCTION
Pregnancy-induced hypertension (PIH) is one of the most common pregnancy complications, affecting approximately 5-7% of all pregnancies. It is also a significant cause of maternal and fetal morbidity and mortality (1). The incidence of PIH in India ranges from 5% to 15% (2). The most common immediate maternal complications of PIH are eclampsia, oligohydramnios, accidental hemorrhages, disseminated intravascular coagulation, and hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome. Remote complications include residual hypertension, recurrent preeclampsia, and chronic renal failure (3). The most common fetal complications of PIH are intra uterine growth retardation, intra uterine death, prematurity, and asphyxia. Many hematological changes are seen in association with PIH, thrombocytopenia being the most common (4,6). Changes are also seen in peripheral smear, coagulation profile, and liver enzymes. This study was done to aid clinicians in early detection, monitoring, and management of cases with PIH.
Preeclampsia develops a variety of hematologic aberrations, which affect the outcome of the patients. In such cases, supportive therapy can be initiated to prevent maternal and neonatal morbidity and mortality. From the standpoint of prevention, preeclampsia has remained a challenge for obstetricians. Various strategies have been proposed to reduce the perinatal effects of preeclampsia. This can be achieved by early diagnosis of preeclampsia simply via assessment of blood coagulation profile (7,9). Complete blood count, urine examination, and liver function tests performed to identify platelet abnormalities, red cell abnormality, and to detect patients who progress to HELLP syndrome.
This study was done to compare hematological indices in patients with gestational hypertension, mild preeclampsia, severe preeclampsia and eclampsia to identify early detection of the disease, and its management to reduce morbidity and mortality of mother and fetus.
MATERIALS AND METHODS
This study was done in the Department of Pathology, Narayana Medical College and Hospital, Nellore, India. This was a prospective study conducted from October 2013 to September 2015 on 114 antenatal PIH cases. Inclusion criteria were >20 weeks of gestation, blood pressure (BP) of >140/90 mmHg, and ≥1+ proteinuria. Previously known cases of hypertension, bleeding disorders, and preeclampsia superimposed on a known case of essential hypertension were excluded. All patients with coexisting medical, surgical or gestational conditions were excluded. The number of patients recruited in this study was calculated using the formula N = Z2P (1-P)/d2 where N is the sample size, Z is the statistic corresponding to level of confidence, P is expected prevalence, and d is precision (10). Considering 95% confidence interval, expected prevalence of 10.3% (11), and absolute precision of 5%, the required sample size was calculated as 114. All samples were collected with the support of the Department of OBG, Narayana Medical College, India.
Data collection
Preliminary data of patients with PIH were collected at admission, coded, and recorded into a master chart. The patients were followed up until perinatal period for final diagnosis and evaluation of disease progression. Venous blood samples were collected in EDTA tubes for hematological profile, in sodium citrate tube for coagulation studies, and in plain tubes for biochemical analysis. The hematological parameters were assessed using an autoanalyzer (LH 780, Beckman Coulter, USA). Erythrocyte sedimentation rate (ESR) was determined using disposable Westergren’s tubes. Automated blood coagulation analyzer (IL ACL 7000 Coagulation Analyzer, Diamond Diagnostics Inc. USA) and the biochemical analysis using automated analyzer (Human Humastar 600 Chemistry Analyzer, Diamond Diagnostics Inc. USA). The findings were recorded as per the proforma. The master chart was prepared having preliminary data (hospital ID, age, gravid status, and BP) as well as hematological and biochemical values. The cases were categorized as gestational hypertension (BP: 140/90 mmHg, no proteinuria), mild preeclampsia (BP: 140/90 – 160/110 mmHg, proteinuria+), severe preeclampsia (BP: >160/110 mmHg), and eclampsia (BP: >140/90 mmHg, seizures+). Various parameters and peripheral smear examinations were studied among these groups.
Statistical analysis
Data were analyzed using the Chi-square test. Statistical analysis was carried out in SPSS (version 22), and p-values less than 0.05 were considered as statistically significant.
RESULTS
Of 114 subjects, 35 were categorized as gestational hypertension, 33 as mild preeclampsia, 40 as severe preeclampsia, and six as eclampsia.
Eight patients (7.01%) consisting of seven cases of severe preeclampsia and one case of eclampsia progressed to HELLP syndrome. As shown in figure 1, the peripheral smear examination showed schistocytes and anisopoikilocytosis in these cases.
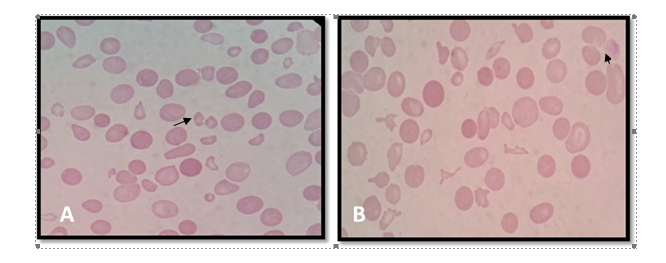
Table 1. Comparison of various parameters between patients with different forms of PIH
*Statistically significant difference (p<0.05).
Table 2. Comparison of various parameters between patient with HELLP syndrome and those without HELLP syndrome
DISCUSSION
Preeclampsia is one of the leading causes of maternal and fetal morbidity and mortality. After active research for many years, the etiology of PIH remains unclear. Evidence suggests that there were several underlying causes for endothelial dysfunction such as hypertension, proteinuria, and edoema, as well as preeclampsia (12). In the present study, important hematological, coagulation, and biochemical values were compared among various groups of PIH. The frequency of patients with gestational hypertension, mild preeclampsia, severe preeclampsia, and eclampsia was 30.7%, 28.9%, 35.1%, and 5.3%, respectively. The majority of patients were in the 20-30 years age group. The present study showed that primigravid patients were equally affected with hypertensive disorders in pregnancy as multigravid patients.
In normal pregnancy, there is an increase in erythropoietic activity, but due to increase in plasma volume, there is a fall in hemoglobin concentration. Normal physiological changes also affect the hematological parameters during pregnancy; hence, maternal anemia is common (12). The percentage of patients with anemia increased with disease severity. We detected a significant decrease in hemoglobin levels with the increase in disease severity. In the present study, 33.3% of the subjects had anemia. The mean hemoglobin concentration was 10.6 g/dl with a standard deviation of 2.12, which is almost comparable to the values reported by Navamar et al. (13) and Alisi et al. (14).
In a study by Monteiro et al., hemoglobin and platelets levels were significantly decreased in cases with preeclampsia (12). In our study, there was a significant reduction in the platelet count as PIH progressed. Platelet count variations in pregnant women with PIH may be due to increased consumption with decreased life span and increased aggregation caused by increased levels of thromboxane A2 in placental circulation (15). Compared to previous studies (13, 14), we obtained higher mean platelet counts, which might be related to the inclusion of patients with gestational hypertension in whom hematological changes are more subtle.
In the present study, 42.1% of patients had thrombocytopenia. Similar studies by Burrows RF and Kelton JG (16) reported prevalence rates of 50% and 34%, respectively.
The mean platelet count in the present study (191 + 84 x 109cells/L) was similar to that in previous studies (13, 17, 18). In our study, ESR increased in few patients, but no ESR rise was seen with increase in disease severity. The mean ESR value in the present study was 71.85 (mm/hr), which is higher than the value reported by Monteiro et al. (57.87(mm/hr)) (12). In normal pregnancy, ESR may rise due to increased fibrinogen and globulin levels and reduced blood viscosity. In our study, eight cases (7.01%) had features of HELLP syndrome. A similar incidence rate (6%) was found in a study done by Sultana et al. (2). However, some studies reported much higher incidence rates (18, 19).
In this study, 27.19% of patients showed increased PT levels, and PT values observed to be increased significantly according to the increased disease severity. Similar to our findings, Shetty et al. reported a significant increase in PT levels in PIH cases (20).
In this study, the mean PT values in cases with mild preeclampsia, severe preeclampsia, and eclampsia were 12.80 + 1.11, 13.02 + 1.53, and 13.61 + 1.33 seconds, respectively. This indicates that PT value increases significantly as PIH progresses. The mean PT values obtained in different forms of the disease were comparable to the values reported by Nirmala et al. (21) and Chauhan et al. (22).
There was a significant increase in aPTT with increase in the severity of the disease. In addition, 20.17% of the patients had increased aPTT level. The mean aPTT in this study was 33.62 seconds, which is similar to the value reported by Dave et al. (23) and Orlikowski et al (24). The mean aPPT was varied in the studies conducted by Chaware K et al (18), Nirmala et al (21) and Chauhan et al (22).
When compared with normal pregnancy, there is a significant increase in hepatic enzyme activity in preeclampsia. Liver damage adversely affects protein metabolism, which in turn could affect erythropoiesis. Reduction in red blood cell and platelet count may be a consequence of liver damage (14).
In the present study, the mean SGOT and SGPT levels were 39.58 ± 32.57 IU/L and 26.75 ± 25.85 IU/L, respectively, which are similar to the levels reported by Munazza et al. (25) and Bhowmik et al. (26). Our study identified significant increase in SGOT/AST and SGPT/ALT ratio as the disease progressess.
The levels of urea, creatinine, and uric acid were increased in patients with PIH when compared to normal individuals, indicating impaired renal function in PIH. These findings are in agreement with findings of some previous studies (12, 14, 27). Uric acid is one of the most sensitive indicators of disease severity in PIH and can help in monitoring disease progression. In preeclampsia, uric acid level has been known to be increased and correlates with maternal and fetal morbidity (20). Wake et al. claimed that plasma uric acid level could help to predict development of eclampsia in subjects with preeclampsia (28). Hawkins et al. reported hyperuricemia as an important finding in PIH because it predicts risk of adverse fetal outcomes, even in women with gestational hypertension without any other features of preeclampsia (29). Mustaphi et al. found a strong positive correlation between levels of serum uric acid and severity of PIH (30).
The present study showed that there is significant increase in the Uric acid levels as the disease progresses, which was comparable to other studies (12, 31).
Some variables such as aPTT, SGOT, and urea levels were higher in cases with HELLP syndrome. In the present study, serum SGOT levels were higher in HELLP cases compared with non-HELLP PIH cases, which is consistent with findings of Shetty et al. (20).
CONCLUSION
Hematological changes such as anemia, thrombocytopenia, and deranged coagulation profile as well as altered liver and renal parameters are seen in PIH. The degree of thrombocytopenia, anemia, and deranged parameters increases as the disease progresses from gestational hypertension to eclampsia. Changes in the liver and renal parameters were prominent in cases of HELLP syndrome, which can be used for earlier identification of these patients. The results show that repeated blood test could help in careful monitoring, early detection, and appropriate management of PIH in order to reduce risk of morbidity and mortality.
ACKNOWLEDGEMENTS
None.
DECLARATIONS
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics approvals and consent to participate
The study protocol was approved by the Institutional Ethics Committee, Naryana Medical College, Nellore, A.P, India (IEC/2014/Pathology.01). Informed consent was obtained after explaining the study procedures.
Conflict of interest
The authors declare that there is no conflict of interest regarding publication of this article
Pregnancy-induced hypertension (PIH) is one of the most common pregnancy complications, affecting approximately 5-7% of all pregnancies. It is also a significant cause of maternal and fetal morbidity and mortality (1). The incidence of PIH in India ranges from 5% to 15% (2). The most common immediate maternal complications of PIH are eclampsia, oligohydramnios, accidental hemorrhages, disseminated intravascular coagulation, and hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome. Remote complications include residual hypertension, recurrent preeclampsia, and chronic renal failure (3). The most common fetal complications of PIH are intra uterine growth retardation, intra uterine death, prematurity, and asphyxia. Many hematological changes are seen in association with PIH, thrombocytopenia being the most common (4,6). Changes are also seen in peripheral smear, coagulation profile, and liver enzymes. This study was done to aid clinicians in early detection, monitoring, and management of cases with PIH.
Preeclampsia develops a variety of hematologic aberrations, which affect the outcome of the patients. In such cases, supportive therapy can be initiated to prevent maternal and neonatal morbidity and mortality. From the standpoint of prevention, preeclampsia has remained a challenge for obstetricians. Various strategies have been proposed to reduce the perinatal effects of preeclampsia. This can be achieved by early diagnosis of preeclampsia simply via assessment of blood coagulation profile (7,9). Complete blood count, urine examination, and liver function tests performed to identify platelet abnormalities, red cell abnormality, and to detect patients who progress to HELLP syndrome.
This study was done to compare hematological indices in patients with gestational hypertension, mild preeclampsia, severe preeclampsia and eclampsia to identify early detection of the disease, and its management to reduce morbidity and mortality of mother and fetus.
MATERIALS AND METHODS
This study was done in the Department of Pathology, Narayana Medical College and Hospital, Nellore, India. This was a prospective study conducted from October 2013 to September 2015 on 114 antenatal PIH cases. Inclusion criteria were >20 weeks of gestation, blood pressure (BP) of >140/90 mmHg, and ≥1+ proteinuria. Previously known cases of hypertension, bleeding disorders, and preeclampsia superimposed on a known case of essential hypertension were excluded. All patients with coexisting medical, surgical or gestational conditions were excluded. The number of patients recruited in this study was calculated using the formula N = Z2P (1-P)/d2 where N is the sample size, Z is the statistic corresponding to level of confidence, P is expected prevalence, and d is precision (10). Considering 95% confidence interval, expected prevalence of 10.3% (11), and absolute precision of 5%, the required sample size was calculated as 114. All samples were collected with the support of the Department of OBG, Narayana Medical College, India.
Data collection
Preliminary data of patients with PIH were collected at admission, coded, and recorded into a master chart. The patients were followed up until perinatal period for final diagnosis and evaluation of disease progression. Venous blood samples were collected in EDTA tubes for hematological profile, in sodium citrate tube for coagulation studies, and in plain tubes for biochemical analysis. The hematological parameters were assessed using an autoanalyzer (LH 780, Beckman Coulter, USA). Erythrocyte sedimentation rate (ESR) was determined using disposable Westergren’s tubes. Automated blood coagulation analyzer (IL ACL 7000 Coagulation Analyzer, Diamond Diagnostics Inc. USA) and the biochemical analysis using automated analyzer (Human Humastar 600 Chemistry Analyzer, Diamond Diagnostics Inc. USA). The findings were recorded as per the proforma. The master chart was prepared having preliminary data (hospital ID, age, gravid status, and BP) as well as hematological and biochemical values. The cases were categorized as gestational hypertension (BP: 140/90 mmHg, no proteinuria), mild preeclampsia (BP: 140/90 – 160/110 mmHg, proteinuria+), severe preeclampsia (BP: >160/110 mmHg), and eclampsia (BP: >140/90 mmHg, seizures+). Various parameters and peripheral smear examinations were studied among these groups.
Statistical analysis
Data were analyzed using the Chi-square test. Statistical analysis was carried out in SPSS (version 22), and p-values less than 0.05 were considered as statistically significant.
RESULTS
Of 114 subjects, 35 were categorized as gestational hypertension, 33 as mild preeclampsia, 40 as severe preeclampsia, and six as eclampsia.
Eight patients (7.01%) consisting of seven cases of severe preeclampsia and one case of eclampsia progressed to HELLP syndrome. As shown in figure 1, the peripheral smear examination showed schistocytes and anisopoikilocytosis in these cases.

Figure 1. Hemolytic picture with many schistocytes (A) and marked anisopoikilocytosis with schistocytes (B)
As shown in table 1, the mean levels of hemoglobin (Hb), platelets, prothrombin time (PT), international normalized ratio (INR), partial thromboplastin time (aPTT), aspartate transaminase (SGOT), alanine transaminase (SGPT), alkaline phosphatase (ALP), creatinine, and uric acid differed significantly between patients in different groups. As shown in table 2, the level of Hb, PC, SGOT, SGPT, and urea differed significantly between cases with HELLP syndrome and those without HELLP syndrome (p≤0.05).Table 1. Comparison of various parameters between patients with different forms of PIH
| Parameter | Gestational hypertension | Mild preeclampsia | Severe preeclampsia | Eclampsia | p-value |
| Hemoglobin (g/dl) | 10.8±1.8 | 10.4±1.9 | 10.8±2.4 | 8.8±1.8 | 0.045* |
| Platelets (x 109cells/L) | 216±72 | 190±90 | 176±84 | 115±30 | 0.008* |
| Erythrocyte sedimentation rate (mm/hr) | 65.91±27.2 | 75.6±31.26 | 73.9±32.8 | 72±22.44 | 0.28 |
| Prothrombin time (seconds) | 13.44±1.27 | 12.8±1.11 | 13.02±1.53 | 13.61±0.93 | 0.05* |
| International normalized ratio | 1.03±0.10 | 1.1±0.2 | 1.14±0.74 | 1.22±0.28 | 0.05* |
| Partial thromboplastin time(seconds) | 33.02±6.42 | 33.05±5.22 | 33.61±6.85 | 40.15±4.17 | 0.03* |
| Aspartate transaminase (IU/L) | 27.68±13.63 | 27.9±14.09 | 59.22±8.35 | 42.33±8.73 | 0.009* |
| Alanine transaminase (IU/L) | 20.05±11.17 | 20.3±10.25 | 37.9±39.13 | 27±7.87 | 0.01* |
| Alkaline phosphatase(IU/L) | 492.37±212.46 | 456.27±198.7 | 502.42±228.23 | 470.16±213.15 | 0.02* |
| Urea(mg/dL) | 16.8±6.66 | 18.38±7.3 | 28.23±16.83 | 30.61±12.21 | 0.07 |
| Creatinine (mg/dL) | 0.75±0.24 | 0.91±0.36 | 0.94±0.37 | 1.31±0.64 | 0.04* |
| Uric acid(mg/dL) | 4.78±1.05 | 4.83±1.49 | 6.11±1.99 | 7.13±3.19 | 0.001* |
*Statistically significant difference (p<0.05).
Table 2. Comparison of various parameters between patient with HELLP syndrome and those without HELLP syndrome
| Parameter | Cases with HELLP syndrome | Cases without HELLP syndrome | p-value |
| Hemoglobin (g/dl) | 9.03±2.77 | 10.63±2.12 | 0.0464* |
| Erythrocyte sedimentation rate (mm/hr) | 81.25±25.8 | 71.85±30.16 | 0.3931 |
| Platelets (x 109cells/L) | 130±90 | 191±84 | 0.0449* |
| Prothrombin time (sec) | 13.9±1.4 | 13.12±1.33 | 0.1137 |
| Partial thromboplastin time (sec) | 36.16±9.19 | 33.62±6.3 | 0.290 |
| Aspartate transaminase (IU/L) | 113.5±157.7 | 39.58±32.37 | 0.0001* |
| alanine transaminase (IU/L) | 46.7±51.9 | 26.75±25.85 | 0.05* |
| Alkaline phosphatase (IU/L) | 498±206.32 | 484.28±213.15 | 0.8607 |
| Urea (mg/dl) | 34.16±24.6 | 22±12.75 | 0.0178* |
| Creatinine (mg/dl) | 1.06±0.48 | 0.89±0.37 | 0.22 |
| Uric Acid (mg/dl) | 5.59±2.3 | 5.39±1.82 | 0.76 |
DISCUSSION
Preeclampsia is one of the leading causes of maternal and fetal morbidity and mortality. After active research for many years, the etiology of PIH remains unclear. Evidence suggests that there were several underlying causes for endothelial dysfunction such as hypertension, proteinuria, and edoema, as well as preeclampsia (12). In the present study, important hematological, coagulation, and biochemical values were compared among various groups of PIH. The frequency of patients with gestational hypertension, mild preeclampsia, severe preeclampsia, and eclampsia was 30.7%, 28.9%, 35.1%, and 5.3%, respectively. The majority of patients were in the 20-30 years age group. The present study showed that primigravid patients were equally affected with hypertensive disorders in pregnancy as multigravid patients.
In normal pregnancy, there is an increase in erythropoietic activity, but due to increase in plasma volume, there is a fall in hemoglobin concentration. Normal physiological changes also affect the hematological parameters during pregnancy; hence, maternal anemia is common (12). The percentage of patients with anemia increased with disease severity. We detected a significant decrease in hemoglobin levels with the increase in disease severity. In the present study, 33.3% of the subjects had anemia. The mean hemoglobin concentration was 10.6 g/dl with a standard deviation of 2.12, which is almost comparable to the values reported by Navamar et al. (13) and Alisi et al. (14).
In a study by Monteiro et al., hemoglobin and platelets levels were significantly decreased in cases with preeclampsia (12). In our study, there was a significant reduction in the platelet count as PIH progressed. Platelet count variations in pregnant women with PIH may be due to increased consumption with decreased life span and increased aggregation caused by increased levels of thromboxane A2 in placental circulation (15). Compared to previous studies (13, 14), we obtained higher mean platelet counts, which might be related to the inclusion of patients with gestational hypertension in whom hematological changes are more subtle.
In the present study, 42.1% of patients had thrombocytopenia. Similar studies by Burrows RF and Kelton JG (16) reported prevalence rates of 50% and 34%, respectively.
The mean platelet count in the present study (191 + 84 x 109cells/L) was similar to that in previous studies (13, 17, 18). In our study, ESR increased in few patients, but no ESR rise was seen with increase in disease severity. The mean ESR value in the present study was 71.85 (mm/hr), which is higher than the value reported by Monteiro et al. (57.87(mm/hr)) (12). In normal pregnancy, ESR may rise due to increased fibrinogen and globulin levels and reduced blood viscosity. In our study, eight cases (7.01%) had features of HELLP syndrome. A similar incidence rate (6%) was found in a study done by Sultana et al. (2). However, some studies reported much higher incidence rates (18, 19).
In this study, 27.19% of patients showed increased PT levels, and PT values observed to be increased significantly according to the increased disease severity. Similar to our findings, Shetty et al. reported a significant increase in PT levels in PIH cases (20).
In this study, the mean PT values in cases with mild preeclampsia, severe preeclampsia, and eclampsia were 12.80 + 1.11, 13.02 + 1.53, and 13.61 + 1.33 seconds, respectively. This indicates that PT value increases significantly as PIH progresses. The mean PT values obtained in different forms of the disease were comparable to the values reported by Nirmala et al. (21) and Chauhan et al. (22).
There was a significant increase in aPTT with increase in the severity of the disease. In addition, 20.17% of the patients had increased aPTT level. The mean aPTT in this study was 33.62 seconds, which is similar to the value reported by Dave et al. (23) and Orlikowski et al (24). The mean aPPT was varied in the studies conducted by Chaware K et al (18), Nirmala et al (21) and Chauhan et al (22).
When compared with normal pregnancy, there is a significant increase in hepatic enzyme activity in preeclampsia. Liver damage adversely affects protein metabolism, which in turn could affect erythropoiesis. Reduction in red blood cell and platelet count may be a consequence of liver damage (14).
In the present study, the mean SGOT and SGPT levels were 39.58 ± 32.57 IU/L and 26.75 ± 25.85 IU/L, respectively, which are similar to the levels reported by Munazza et al. (25) and Bhowmik et al. (26). Our study identified significant increase in SGOT/AST and SGPT/ALT ratio as the disease progressess.
The levels of urea, creatinine, and uric acid were increased in patients with PIH when compared to normal individuals, indicating impaired renal function in PIH. These findings are in agreement with findings of some previous studies (12, 14, 27). Uric acid is one of the most sensitive indicators of disease severity in PIH and can help in monitoring disease progression. In preeclampsia, uric acid level has been known to be increased and correlates with maternal and fetal morbidity (20). Wake et al. claimed that plasma uric acid level could help to predict development of eclampsia in subjects with preeclampsia (28). Hawkins et al. reported hyperuricemia as an important finding in PIH because it predicts risk of adverse fetal outcomes, even in women with gestational hypertension without any other features of preeclampsia (29). Mustaphi et al. found a strong positive correlation between levels of serum uric acid and severity of PIH (30).
The present study showed that there is significant increase in the Uric acid levels as the disease progresses, which was comparable to other studies (12, 31).
Some variables such as aPTT, SGOT, and urea levels were higher in cases with HELLP syndrome. In the present study, serum SGOT levels were higher in HELLP cases compared with non-HELLP PIH cases, which is consistent with findings of Shetty et al. (20).
CONCLUSION
Hematological changes such as anemia, thrombocytopenia, and deranged coagulation profile as well as altered liver and renal parameters are seen in PIH. The degree of thrombocytopenia, anemia, and deranged parameters increases as the disease progresses from gestational hypertension to eclampsia. Changes in the liver and renal parameters were prominent in cases of HELLP syndrome, which can be used for earlier identification of these patients. The results show that repeated blood test could help in careful monitoring, early detection, and appropriate management of PIH in order to reduce risk of morbidity and mortality.
ACKNOWLEDGEMENTS
None.
DECLARATIONS
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics approvals and consent to participate
The study protocol was approved by the Institutional Ethics Committee, Naryana Medical College, Nellore, A.P, India (IEC/2014/Pathology.01). Informed consent was obtained after explaining the study procedures.
Conflict of interest
The authors declare that there is no conflict of interest regarding publication of this article
Article Type: Research |
Subject:
Basic medical sciences
References
1. Fatemeh T, Marziyeh G, Nayereh G, Anahita G, Samira T. Maternal and perinatal outcome in nulliparious women complicated with pregnancy hypertension. JPMA. The Journal of the Pakistan Medical Association. 2010 Sep 1;60(9):707. [Google Scholar]
2. Sultana F, Parthiban R, Shariff S. Thrombocytopenia in pregnancy induced hypertension. J Med Sci Health. 2015;1(2):19-24 [DOI] [Google Scholar]
3. Dutta.D.C. Hypertensive Disorders In Pregnancy. In: Hiralal, K (ed.) Textbook of Obstetrics Including . Kolkata, India: Jaypee Brothers Medical Publishers (P) Ltd; 2013.p. 219-240. [DOI]
4. Bora R, Sable C, Wolfson J, Boro K, Rao R. Prevalence of anemia in pregnant women and its effect on neonatal outcomes in Northeast India. The Journal of Maternal-Fetal & Neonatal Medicine. 2014 Jun 1;27(9):887-91. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Khan A, Fahim A, Qureshi A, Nizamani GS, Azmi MA. Pregnancy Induced Hypertension; Assessment of prognostic value of platelet count in women with varying degree. Professional Med J. 2014;21(3):436- 40. [DOI] [Google Scholar]
6. Ratnam SS, Rao KB, Arul kumaran S. Obstetrics and Gynecology for postgraduates, Vol 1. 2nd Edition. Orient Longman, Hydrabad. 1999:60.
7. Schlembach D. Pre-eclampsia--still a disease of theories. Fukushima journal of medical science. 2003;49(2):69-115. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Lescale KB, Eddleman KA, Cines DB, Samuels P, Lesser ML, McFarland JG, Bussel JB. Antiplatelet antibody testing in thrombocytopenic pregnant women. American journal of obstetrics and gynecology. 1996 Mar 1;174(3):1014-8. [View at Publisher] [DOI] [Google Scholar]
9. Karim R, Sacher RA. Thrombocytopenia in pregnancy. Current Hematology Reports. 2004 Mar 1;3(2):128-33. [View at Publisher] [Google Scholar]
10. Dhinwa M, Gawande K, Jha N, Anjali M, Bhadoria AS, Sinha S. Prevalence of hypertensive disorders of pregnancy in India: A systematic review and meta-analysis. J Med Evid. 2021 May 1;2:105-2. [DOI] [Google Scholar]
11. Magee LA, Sharma S, Nathan HL, Adetoro OO, Bellad MB, Goudar S, Macuacua SE, Mallapur A, Qureshi R, Sevene E, Sotunsa J. The incidence of pregnancy hypertension in India, Pakistan, Mozambique, and Nigeria: a prospective population-level analysis. PLoS medicine. 2019 Apr 12;16(4):e1002783. [View at Publisher] [DOI] [PMID] [PMCID] [Google Scholar]
12. Monteiro G, Subbalakshmi NK, Pai SR. Relevance of measurement of hematological parameters in subjects with pregnancy induced hypertension. Nitte University Journal of Health Science. 2014 ;4(1):15 [View at Publisher] [DOI] [Google Scholar]
13. B Namavar Jahromi, SH Rafiee. Coagulation Factors in Severe Preeclampsia. Iranian Red Crescent Medical Journal. 2009; 11(3):321-324. [View at Publisher] [Google Scholar]
14. Alisi PN, Buseri FI, Alisi CS. Some Blood Cell Changes and Alteration in Renal and Hepatic Function in Pre-eclampsia: A Study in Owerri Nigeria. IBRR 2014; 4; 132-139. [View at Publisher] [DOI] [Google Scholar]
15. Madazli R, Benian A, Gümüştaş K, Uzun H, Ocak V, Aksu F. Lipid peroxidation and antoxidants in preeclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1999 Aug 1;85(2):205-8. [View at Publisher] [DOI] [Google Scholar]
16. Burrows RF, Kelton JG. Fetal thrombocytopenia and its relation to maternal thrombocytopenia. N Engl J Med. 1993;329(20):1463-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Mohapatra S, Pradhan BB, Satpathy UK, Mohanty A, Pattnaik JR. Platelet estimation: its prognostic value in pregnancy induced hypertension. Indian J Physiol Pharmacol 2007; 51 (2) : 160- 164. [Google Scholar]
18. Chaware SA, Dhake R, Ingole AS, Bahattare VN, Bhopale KS. Study of Coagulation Profile in Preeclampsia and Eclampsia. International Medical Journal. 2015;2(3):164-70. [Google Scholar]
19. Onisai M, Vladareanu AM, Bumbea H, Ciorascu M, Pop C, Andrei C, Nicolescu A, Voican I, Vasilescu S, Visan L, Adrian II. A study of the hematological picture and of platelet function in preeclampsia-report of a series of cases. Maedica-a Journal of Clinical Medicine. 2009 Dec 1;4(4). [Google Scholar]
20. Shetty J, Rao S, Kulkarni MH. Hematological Changes in Pregnancy-induced Hypertension. International journal of scientific study. 2016;4(5):215-20. [Google Scholar]
21. Nirmala T, Kumar Pradeep L, Vani B R., Murthy Srinivasa V, Geetha RL. Study Of Coagulation Profile In Pregnancy Induced Hypertension. Indian Journal Of Pathology and Oncology;2015;2(1)1-6.
22. Chauhan P, Rawat U, Bisht V, Purohit RC. Comparison of cogulation profile in pre eclamptic and eclamptic patients with normotensive pregnant patients. Journal of Evolution of Medical and Dental Sciences. 2014 Mar 24;3(12):3208-16. [DOI] [Google Scholar]
23. Dave R, Agravat A, Dhruva G, Katara A. Comparative Study of Coagulation Factors in Pre-Eclampsia and Normal Pregnancy. International Journal of Scientific Research;2014;3(4);377-378. [DOI] [Google Scholar]
24. Orlikowski CE, Rocke DA, Murray WB, Gouws E, Moodley J, Kenoyer DG, Byrne S. Thrombelastography changes in pre-eclampsia and eclampsia. British Journal of Anaesthesia. 1996;77(2):157-61. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Munazza B, Raza N, Naureen A, Khan SA, Fatima F, Ayub M, Sulaman M. Liver function tests in preeclampsia. J Ayub Med Coll Abbottabad. 2011;23(4):3-5. [Google Scholar]
26. Bhowmik DK, Akhtari R, Kumar SU, Saha M, Adhikary DK. Alteration of liver function in preeclampsia and eclampsia. Chattagram Maa-O-Shishu Hospital Medical College Journal. 2013 Oct 28;12(3):9-10. [DOI] [Google Scholar]
27. Ganai I, Shazia JS, Parveen N, Arif K, Farooq AG. To Study Biochemical and Hematological Parameters in Pre-Eclampsia; JMSCR; 2014;2(1);315-320.
28. Wakwe VC, Abudu O. Estimation of plasma uric acid in pregnancy induced hypertension (PIH). Afr J Sci. 1999:155-8. [Google Scholar]
29. Hawkins TL, Roberts JM, Mangos GJ, Davis GK, Roberts LM, Brown MA. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG 2012; 119(4):484-92. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Mustaphi R, Gopalan S, Dhaliwal L, Sarkar AK. Hyperuricemia and pregnancy induced hypertension-reappraisal. Indian J Med Sci 1996; 50 (3):68-71. [View at Publisher] [Google Scholar]
31. Sunitha T, Sameera K, Umarani G. Study of Biochemical Changes In Pre-Eclamptic Women; International Journal Of Biological & Medical Research; 2012;3(3):2025-2028. [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).





